Fig. 33.1
a and b Suprasellar craniopharyngioma (sagittal and axial projections) demonstrating the common appearance of this lesion with solid and cystic components. CT imaging (not shown here) commonly demonstrates peripheral calcification
Pituitary adenomas are the second most common lesion in the sellar and parasellar region in children and account for 3% of the total. Prolactinomas and adrenocorticotropic hormone (ACTH) secreting tumors are the most common pituitary adenomas requiring treatment and nonsecretory adenomas are less frequent [4, 7] (see Fig. 33.2).
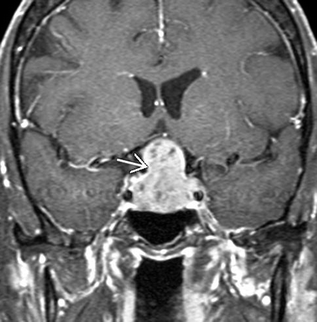

Fig. 33.2
Macroadenoma with suprasellar extension. Classic “snowman” appearance on imaging as the lesson violates the diaphrama sella superiorly. Laterally, the lesion appears to have cavernous sinus invasion with tumor around the carotid arteries bilaterally
Preoperative Evaluation
Except in emergency cases of pituitary apoplexy with visual compromise or cases of acute obstructive hydrocephalus, a complete medical evaluation by a multidisciplinary team is performed before surgical intervention. Specifically, detailed endocrine and ophthalmological evaluation must be performed.
Preoperative endocrine evaluation should include measurement of prolactin, growth hormone, thyroid stimulating hormone (TSH), follicle stimulating hormone (FSH), leutinizing hormone (LH), cortisol, triiodothyrinone (T3), and thyroxine (T4). This evaluation will reveal secretory lesions such as prolactinomas that might be amenable to medical therapy. In addition, baseline hormonal deficits that could cause perioperative problems will be identified and replacement therapy can be instituted. Mild hyperprolactinemia (<200 ng/mL) may occur with suprasellar lesions that do not secrete prolactin but only compress the pituitary stalk (the “stalk effect”) [8, 9].
Baseline ophthalmological evaluation is necessary to assess for preoperative vision deficits that may be caused by the tumors. Although a major goal of pituitary surgery is to preserve vision, sometimes transsphenoidal and transcranial procedures may adversely affect visual acuity. The careful preoperative and postoperative assessment of vision allows for appropriate counseling of patients and families [8, 9].
All patients with sellar pathology typically undergo computed tomography (CT) and should also undergo magnetic resonance imaging (MRI) with and without contrast. The CT helps to define pneumatization of the sinuses and determines if special instrumentation is required to access the sellar region through the nonpneumatized sinuses. CT also delineates the anatomy of the sphenoid sinus including the septa that may be present and their relationship to the carotid impression on either side of the sella. Recognition of these relationships is important to help avoid injury to the carotid arteries. MRI provides better definition of the neural structures and the relationship of the tumor to the optic chiasm, optic nerves, carotid arteries, and the cavernous sinus. The degree of tumor extension into surrounding regions is assessed and can determine which nares is used for transsphenoidal access or from which side of the head the tumor is approached during transcranial resection. High resolution MRI may also better identify the sphenoid septa and their relationship to the cavernous carotid arteries than CT. Identification of the carotid arteries is critical because some patients have paramedian or “kissing” cavernous carotid arteries that prevent the transsphenoidal approach [10]. Not recognizing this variant on preoperative imaging could lead to vascular injury, severe neurovascular morbidity and even mortality.
Redo transsphenoidal surgical procedures require a precise preoperative definition of anatomy since the normal landmarks that allow safe access through this trajectory are no longer present and the risk of injury to the cavernous carotid is higher. This imaging is essential for the computer-assisted, image-guided techniques that are especially valuable in redo cases to allow for a safe operative approach.
Surgical Goals
The aims of surgical resection of sellar and parasellar masses are to relieve compression on surrounding structures, such as the optic chiasm, to attain gross total resection to minimize recurrence and eliminate hormonal hypersecretion, to minimize brain retraction, and to preserve pituitary function [8]. The majority of children with sellar lesions present to medical attention with acute hydrocephalus due obstruction of third and lateral ventricular outflow tracts [2, 11, 12]. Resection of the mass relieves the obstruction and in some cases may obviate the need for long-term CSF diversion by either ventriculoperitoneal shunting and or endoscopic third ventriculostomy. Lesions with rapid growth, necrosis, or hemorrhage may compress the optic chiasm causing precipitous loss of vision and may also impair the blood supply to the pituitary causing “pituitary apoplexy” with loss of normal hormonal function [13–15]. Emergent medical evaluation and surgical decompression is essential to preserve visual acuity and prevent the ensuing hormonal deficiencies such as acute adrenal insufficiency [13–15].
Failure of pharmacologic management of sellar masses with continued growth and mass effect, specifically pituitary adenomas such as prolactinomas warrants surgical intervention [16]. In addition, some patients do not tolerate medical management of their sellar masses and may need surgical intervention. Recurrence of disease after prior radiation and medical management is also an indication for surgical extirpation to prevent further disease progression and neurological decline [15–19].
History of Surgical Treatment of Sellar Tumors
In 1866, Pierre Marie described two adult patients with acromegaly and an enlarged sella and sparked investigations into neuroendocrinology and surgical treatment of pituitary lesions [20]. Initially, the surgical approaches were transcranial, but high mortality rates from infection in the era before antibiotics led surgeons to pursue extracranial access to the sella turcica [21–26]. In 1907, Schloffer performed the first successful transsphenoidal resection of a pituitary lesion without complication [27]. Cushing later used the transnasal transsphenoidal approach to resect 231 pituitary tumors with a 5.6% mortality rate [28]. Cushing later abandoned the transsphenoidal procedures and advocated transcranial approaches to the pituitary, but Dott and Guiot continued to champion the transsphenoidal route [29] The morbidity and mortality rates of patients undergoing transnasal transsphenoidal surgery continued to decrease with advances in anesthesia, medical support, and surgical instrumentation [30]. A major advance was the introduction of the surgical microscope in 1971 by Hardy which markedly improved visualization and the precision of excision through the small access corridor [31].
Surgical Approaches to the Pituitary
The choice of transsphenoidal or transcranial approaches to pituitary lesions is influenced by the extent of tumor invasion within the suprasellar and parasellar space as defined by preoperative neuroimaging and by the experience of the surgeon [9, 32]. The transnasal transsphenoidal is usually the preferred route and is the mainstay of surgical treatment of lesions of the sella turcica [8, 15, 16, 19, 32–35]. Technological advancements such as computer-assisted, image-guided surgery, frameless stereotaxy, fluoroscopy, endoscopy, and extended transsphenoidal approaches allow for less invasive methods to access and attain complete tumor resection. Many series of transnasal transsphenoidal resections report mortality rates of less than 1% [17, 18, 34, 36, 37].
However, some sellar and parasellar masses are not amenable to the transsphenoidal approach due to the intimate association with neurovascular structures, the optic apparatus, and the hypothalamus. Such lesions require transcranial approaches for safe gross total resection. Transsphenoidal resections in children may be more challenging than similar resections in adults because of the lack of pneumatization of the sphenoid sinus and the smaller size of the nares and operative corridor. In these circumstances, surgeon experience is of paramount importance for optimal outcomes.
Transsphenoidal Approaches
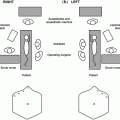
Transsphenoidal resection of sellar tumors spaces may be done via sublabial or transnasal, also known as endonasal, approaches (Fig. 33.3). In 1910, Harvey Cushing was the first neurosurgeon to adopt the sublabial transsphenoidal approach to the sella [28]. Typically the patient is supine with the face parallel to the ceiling and the head approximately 15° above the heart to encourage venous drainage. Flexion of the head can allow for visualization of lesions that extend inferiorly along the clivus while extension of the head allows better visualization of lesions with suprasellar extension. The patient’s head is placed on a horseshoe or Mayfield three point fixation if frameless stereotaxy and intraoperative navigation is used. The endotracheal tube is gently placed to the left of midline and the oropharynx is packed to prevent aspiration of blood. The thigh or the abdomen is also prepared as a donor site for possible fat and fascia grafts. Topical and injection of lidocaine with epinephrine promotes hemostasis and shrinks the nasal turbinates. The upper lip is everted and a sublabial incision is made from one canine fossa to the other down to the maxilla and a subperiosteal dissection superiorly exposes the piriform aperture and rostrum of the maxilla. The floor of the nasal cavity is identified and the mucosa is dissected from the floor with great care along the mesial aspect and carried posteriorly and superiorly to free the mucosa from side of the nasal septum. The quadrangular cartilage is visualized and disarticulated from the vomer and perpendicular plate and mobilized away from the midline. The rostrum and ostia of the sphenoid sinus is identified. The ostia of the sphenoid sinus mark the superior extent of bone removal. A self-retaining Hardy speculum is inserted into this opening in the sphenoid and the operative microscope is brought in. The rostrum or face of the sphenoid is then removed with a combination of ronguers and kerosene punches until the sphenoid septa and mucosa are well visualized. The mucosa is removed to help with hemostasis and prevent postoperative mucocele formation. In children whose sinus is not pneumatized, the bone must be drilled out and intraoperative navigation or fluoroscopy will be necessary to gain access through the sphenoid bone and to the sella.
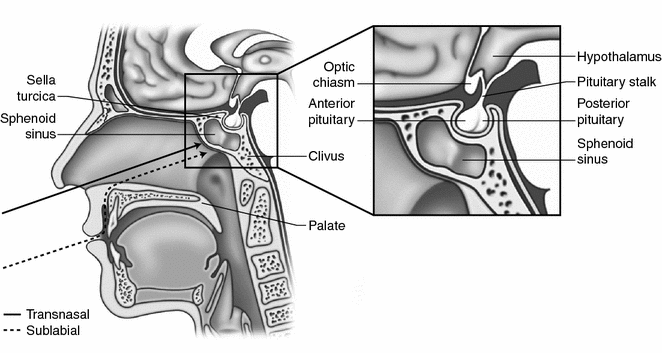

Fig. 33.3
The transphenoidal approach to the suprasellar region is accomplished through either a transnasal or sublabial trajectory. In young children, the translabial approach is preferred as it offers a larger working channel to access the sellar region
Once the sella is identified, the sellar floor is removed posterosuperiorly with microronguers or a drill. Often times, the tumor has already eroded through the floor of the sella and dura may be readily appreciated. The boundaries of the bony removal of the sella are marked by the cavernous sinus laterally and the circular sinus superiorly. The dura is then incised and the mass is resected in a methodical fashion with microinstuments, starting with the floor of the sella first, followed by the lateral extent of the sella. This sequence of resection allows the superior most portion of the tumor to drop into the operative field, driven by pulsations of the intact arachnoid. Care must be taken with resection of the lateral regions of tumor to avoid carotid artery injury. It is important to understand the relationship between the tumor, the normal pituitary gland, and suprasellar arachnoid in order to minimize the risk of a cerebrospinal fluid (CSF) leak and long-term CSF fistula. If a CSF leak with violation of the arachnoid is identified, an autologous fat and fascia graft is harvested from either the abdomen or leg and the fascia graft is placed against the dural opening and the sphenoid sinus is packed with fat to hold the fascia graft in position. A Valsalva maneuver is used to ensure that the graft is in good position. The sublabial incision is then closed with plain catgut sutures, nasal tampons are inserted to aid with hemostasis, and a mustache dressing is applied.
The sublabial approach offers a wider exposure for direct view of the sella and medial portion of both cavernous sinuses [38, 39] while maintaining normal nasal anatomy with retention of the fully intact nasal mucosa and middle turbinates [40, 41]. This can be especially important in Cushing’s disease when extensive inspection of the entire sella may be required to find the typical causative microadenoma [38, 39]. Children also have smaller nasal apertures and the sublabial route provides a wider corridor to access the sella than through the endonasal route [38, 39]. Great caution must be taken opening the Hardy nasal speculum within the sphenoid sinus in children because the thin surrounding bone and narrowness of the sphenoid sinus makes the carotid arteries more vulnerable to injury [2, 9]. Depending on the degree of mucosal injury and the extent of bony resection, there can be postoperative perforations within the nares and associated cosmetic concerns such as a saddle nose-deformity. The biggest disadvantage of the sublabial approach is the resulting paresthesia of the upper lip and incisors [39].
Due to the associated paresthesias experienced with the sublabial approach, the endonasal transsphenoidal approach has been used with greater frequency in those patients whose nares will allow placement of an endonasal speculum [9, 17, 39]. An incision in the posterior nasal septum or a relaxing alar incision may be made in the floor of the nasal cavity to allow easier introduction of the nasal speculum [17]. The patient is positioned and prepped as previously described. A handheld speculum is placed in the nares and the microscope is brought in. The floor of the nasal cavity, the inferior turbinate, and the middle turbinate are identified. An incision is made in the mucosa medial to the middle turbinate abutting the nasal septum and the nasal septum is disarticulated and retracted laterally. A submucosal dissection is then performed on either side of the keel of the nasal septum until the superior sphenoid ostia are identified. The Hardy speculum is then inserted into the nare and the face of the sphenoid is removed along with the mucosa with microronguers and kerosene punches. The remainder of the tumor resection proceeds as previously described.
The endonasal approach has gained widespread favor because it reduces the incidence of numbness of the upper teeth associated with the sublabial approach and avoids potential scarring of the vestibule [17, 39]. The major limitation of this approach is reduced exposure which is a more common issue in children because of their small size [9, 17]. The relaxing alar incision used to gain adequate exposure can cause postoperative bleeding and mucosal irregularity which can bother the patient. This endonasal approach may also result in septal perforations and deviation of the bony septum [9, 17].
Improvements in neuroimaging and intraoperative navigation coupled with technological advances in operative instrumentation allow access to regions of the skull base such as the cavernous sinus, suprasellar cistern, and clivus that were once thought to be accessible only by transcranial approaches. These “extended” transsphenoidal approaches are only useful in patients who have small tumors with limited intradural extension [9, 17]. The rich network of perforators that reside in this region increase the risk of bleeding for larger tumors due to the lack of proximal control and direct visualization [17, 18, 32, 42–50].
Bushe and Halves first reported the use of the endoscope in transsphenoidal surgery in 1978 [51]; however, this technique did not gain widespread acceptance until the mid-1990s when it was used extensively by otolaryngologists in sinus surgery [52]. This approach typically involves only one nostril. The endoscope can be held by either an assistant or can be held in the surgeon’s nondominant hand, and surgical instruments can be held in the surgeon’s dominant hand [53]. Once the anterior sphenoidectomy is performed the endoscope is introduced, or it can be introduced to perform the sphenoidectomy depending on the surgeon’s preference. At this point the endoscope is mounted freeing both hands of the surgeon to proceed ahead with resection of the tumor. Others introduce the microscope when tumor resection ensues. The endonasal endoscopic approach also offers a less traumatic route for the resection of sellar and parasellar lesions, especially in the pediatric population [2, 52]. The microscope offers stereoscopic three-dimensional visualization, illumination, and magnification of the operative field. Endoscopy allows for a larger and closer view of the surgical field, particularly providing better anatomical detail and visualization of any residual pathology, and a lower complication rate. The microscope and endoscope can be used to complement each other to provide superior results than either method alone. The use of the endoscope is technically challenging, especially in the pediatric population with a small pituitary fossa and the absence of sphenoid sinus aeration. This technique provides a narrower working corridor and avoids the resection of the nasal turbinates and septum. Transcranial Approaches.
Although Cushing was a pioneer in transsphenoidal surgery, he later preferred transcranial approaches and his influence made this a common approach through the 1950s [54, 55]. As transsphenoidal approaches came into favor later in the twentieth century, indications for transcranial approaches to the sellar region became limited. Cases where the transsphenoidal approach is contraindicated include presence of sphenoid sinusitis and paramedian carotid arteries. Patients who have extensive suprasellar and parasellar extension of tumor are also good candidates for transcranial approaches that reduce the risk of injury and associated morbidity [56]. The two most common transcranial approaches to gain access to the sellar, parasellar, and suprasellar regions are the pterional (fronto-temporal) and the anterior subfrontal approach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree