Fig. 20.1
Gonocyte transformation in the first year of life is an incomplete process, with many germ cells failing to transform and then undergoing apoptosis. It is quite possible that this is a sorting mechanism to select the “best” germ cells to become spermatogenic stem cells
Postnatal Development in Cryptorchidism
In babies with cryptorchidism the postnatal hormone production by the testis is suppressed [15, 16], and the development of germ cells is interrupted at the first step postnatally, transformation of the gonocytes into AD-spermatogonia [17, 18]. Whether this failure is related to innate genetic errors in the cryptorchid testis, or secondary to the abnormally high postnatal temperature of the misplaced organ, is unknown, but a secondary anomaly seems more likely [19]. Moreover, the entire management of undescended testes is based precisely on this premise, that the postnatal dysfunction is secondary to the position, and hence reversible with orchidopexy.
Many germ cells that fail to transform into AD-spermatogonia, probably secondary to the abnormal temperature interfering with cellular function, also fail to undergo apoptosis. This is likely to leave a cohort of gonocytes in the testicular tubules well beyond their normal time, and we suspect that they are the origin of the subsequent carcinoma-in-situ (CIS) cells after puberty, as they carry the same enzymatic markers [20]. The CIS cells are thought to be the origin of seminomas in previously cryptorchid men [21–23].
Timing of Orchidopexy
Surgical correction of testicular malposition is based on the assumption that postnatal dysfunction is secondary to abnormal temperature, and hence reversible with orchidopexy. Given that gonocyte transformation to AD-spermatogonia is deranged, orchidopexy should aim to correct this and hence should be performed within the first year of life. The exact optimal time remains unknown.
In the first 3 months after birth in term babies about half the testes not in the scrotum initially will descend. Beyond 12 weeks after term delayed descent becomes rare, therefore timing of orchidopexy should take this into account [24–26]. Also, there is widespread agreement amongst anaesthetists that elective surgery should be delayed until about 6 months, when day surgery is known to be safe, and it is well beyond the neonatal period where there remain some doubts about the long-term effects of general anaesthesia. Taken together, these factors lead to 6 months being our current recommended age for orchidopexy for congenital cryptorchidism, which allows for delayed testicular descent and hopefully is early enough to permit postnatal germ cell maturation before it is too late.
Unfortunately, there are no randomised, controlled trials showing the value of orchidopexy at 6 months of age, given the long lag-time before fertility can be measured. There are, however, promising recent studies using testicular growth (measured by ultrasonography) as a very early surrogate for later fertility. By measuring testicular volume, Kollin et al. [27] have shown that surgery at 9 months is significantly better than at 3 years of age.
The gradual reduction in recommended age for orchidopexy over the past few decades has revealed a second group of cryptorchid testes that present later in childhood. We now understand that these are probably acquired rather than congenital, and that they have some differences in aetiology and outcome.
Acquired Cryptorchidism
The first reports of possible acquired cryptorchidism appeared in the 1970s [28] but more widespread recognition of this phenomenon did not occur until the 1990s [29–32], when a group of children undergoing surgery at 5–10 years of age appeared despite an earlier recommended age for orchidopexy. Initially it was assumed that this was caused by delayed diagnosis of congenital cryptorchidism, however, it gradually became more obvious that in fact it was more likely the first presentation of acquired cryptorchidism.
There is now general acceptance that acquired cryptorchidism is not only a real entity, but also that it is quite common [33]. In some hospital audits, it makes up about half of all the orchidopexies being performed [34]. Prior to recognition of this variant, such children were probably diagnosed as having “retractile testes” [35–37]. The aetiology of acquired cryptorchidism remains uncertain, but may be related to minor androgen deficiency and failure of the processus vaginalis to obliterate completely after birth [30]. Persistence of a fibrous remnant of the processus vaginalis is likely to prevent elongation of the spermatic cord, so that as the boy grew and the scrotum become further away from the external inguinal ring, the testis would be left behind and appear to gradually ascend out of the scrotum [29] (see Fig. 19.5 in Chap. 19).
As the testis is intra-scrotal during the first year, we might anticipate that as a result of normal postnatal germ cell maturation, the gonocytes should have disappeared, and that the AD-spermatogonia should be present in adequate numbers. This would be predicted to lead to a low risk of malignancy, if abnormal gonocytes really are the origin of seminomas in previously cryptorchid testes.This is consistent with recent long-term follow-up of men with previous acquired cryptorchidism, showing no increased incidence of malignancy, but some suppression of fertility [38]. Given the potential prognosis of acquired cryptorchidism, many authors recommend orchidopexy once the testis no longer reaches the scrotum.
Indications for Treatment
The scrotum is a specialised, low-temperature environment which allows optimal postnatal development of the gonocytes and postpubertal spermatogenesis [39]. The temperature of the scrotum is 33 °C, which means that intraabdominal testes are 4 °C away from optimal physiology and intracellular functioning, which is the likely reason for secondary dysplasia. Temperature-dependent dysgenesis is proposed to cause progressive loss of the spermatogonia, leading to subsequent poor sperm counts, while any residual gonocytes might eventually mutate into a seminoma.
Successful surgical placement of the testis into the scrotum is dependent not only on the assumption that the damage is secondary rather than a primary disorder of the testis, but also that early intervention can prevent and/or reverse this. Additionally orchidopexy aims to overcome the cosmetic deformity.
Hormonal treatment has been controversial, since it was first introduced in the 1940s. In the 1970s and 1980s, gonadotrophins or analogues of gonadotrophin-releasing hormone were recommended for making the testis descend, but randomised, controlled trials have failed to substantiate the initial claims of success [40]. In recent years, hormone therapy has made a comeback as a way of improving germ cell development after orchidopexy [41].
Surgical Management
The choice of surgical approach depends on the type or location of the cryptorchid testis. Congenital undescended testes can be categorised into palpable and impalpable, with standard inguinal orchidopexy for the former and laparoscopy for the latter. For acquired cryptorchidism the scrotal approach described by Bianchi is useful [45], and some surgeons would recommend this approach for many palpable congenital undescended testes.
Inguinal Orchidopexy
After an inguinal incision the testis within the tunica vaginalis is mobilised and the inguinal canal opened (Fig. 20.2). The distal attachment of the gubernaculum is divided and the cremaster muscle stripped off the spermatic cord (Fig. 20.3). The vas deferens and the testicular vessels are carefully separated from the processus vaginalis, (Fig. 20.4) which is transfixed and ligated at the internal inguinal ring (Fig. 20.5). The retroperitoneal space cranial to the internal ring is opened up so that the vas and particularly the vessels can be freed up from fibrous adhesions preventing stretching out their length. This is usually enough to achieve adequate length, but occasionally the cord structures need to be brought down medial to the inferior epigastric vessels, either by buttonholing the transversalis fascia or by ligating and dividing the vessels to pull the cord medially (Fig. 20.6).
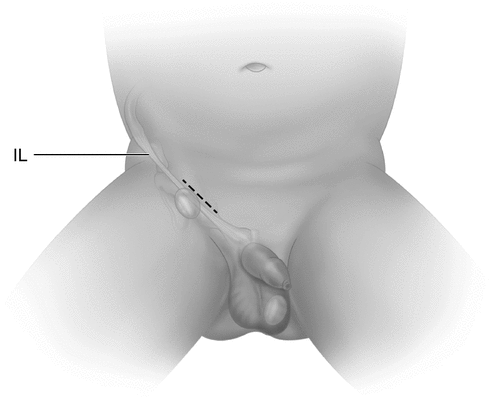
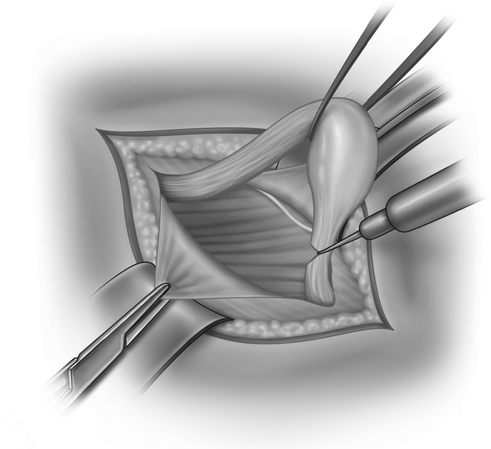
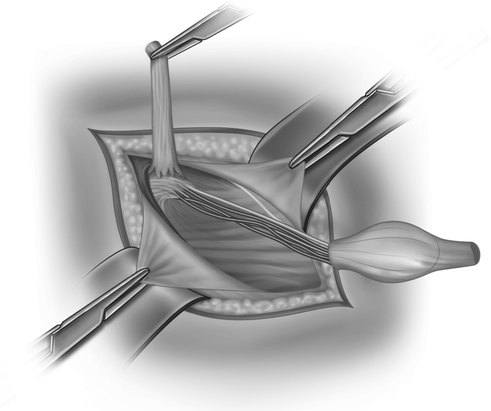
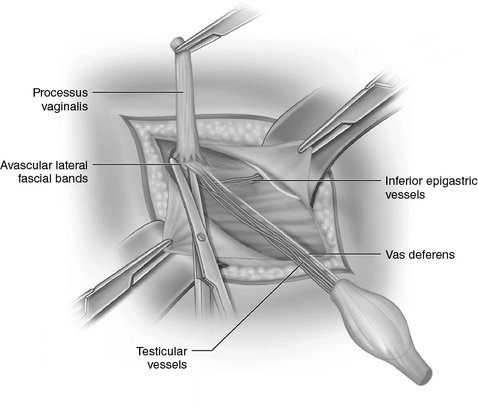
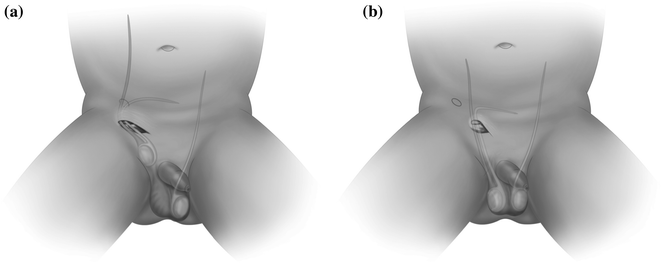

Fig. 20.2
Inguinal skin crease incision

Fig. 20.3
The cremaster muscle fibres are stripped off the cord, allowing the gubernacular attachment to be divided

Fig. 20.4
The sac is stretched over the index finger while dissecting forceps gently brush off the other cord structures

Fig. 20.5
Once separated from the vas deferens and vessels, the sac is transfixed and ligated at the internal inguinal ring

Fig. 20.6
a Transposition of the cord structures medial to the inferior epigastric vessels (known as the Prentiss manoeuvre) allows the spermatic cord to take a more direct path to the external ring. b The Prentiss manoeuvre increases the relative length of the spermatic cord by at least 1cm, even in infants.
The subcutaneous path to the scrotum is made by blunt dissection, usually with a finger (Fig. 20.7), and the scrotum is opened to develop a dartos pouch large enough for the testis. An artery forceps is passed through the scrotal incision and pushed up to the inguinal incision, guided by the finger. Taking care not to twist the spermatic cord, the testis is pulled down to the scrotal incision (Fig. 20.8). The tunica vaginalis is opened and any appendages removed (this prevents exploration for acute scrotum later in childhood) and the testis sutured to the scrotal septum. After closure of the inguinal canal and Scarpa’s fascia, both skin incisions are closed.
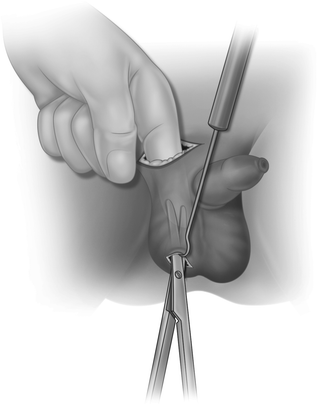
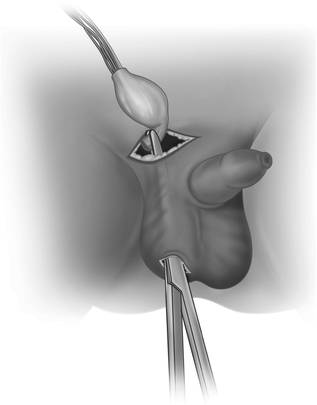

Fig. 20.7
A finger is used to create a passage to the scrotum by blunt dissection and the scrotum is opened

Fig. 20.8
After ensuring no twist in the spermatic vessels, the testis is pulled to the scrotum
Laparoscopic Orchidopexy
The laparoscope is inserted through an umbilical trocar and the presence and location of the testis identified. In a significant percentage of impalpable testes, this documents blind-ending vessels in the retroperitoneum, consistent with the vanishing testis, likely caused by perinatal torsion. This may be associated with an atrophic remnant, which is usually a tiny nodule in the scrotum. Whether or not this needs excision via a small scrotal incision remains controversial.
When the testis is found at laparoscopy, the key determinant for treatment is the proximity of the gonad to the internal inguinal ring. ‘Peeping’ or ‘Peep-a-Boo’ testes sitting just inside the internal ring are usually amenable to straightforward orchidopexy, with or without some laparoscopic mobilisation (Fig. 20.9).
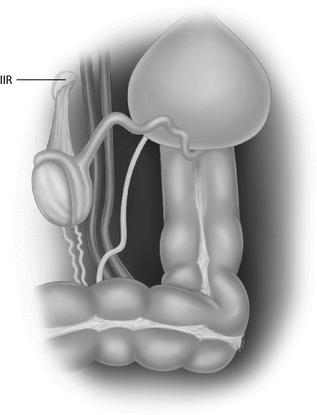

Fig. 20.9




The laparoscopic appearance of an intra-abdominal testis. The canal is often open and contains a gubernacular remnant. The testis is proximal to the ring on a short mesorchium with obvious vas and vessels
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree