Primary liver tumors
Hepatocellular carcinoma
Cholangiocarcinoma
Hilar cholangiocarcinoma
Gallbladder cancer
Metastatic liver disease
Colorectal cancer
Neuroendocrine tumors
Non-colorectal non-neuroendocrine tumors
The oncologic appropriateness of surgical resection varies according to the tumor biology. The presence of multiple lesions or extrahepatic disease in patients with hepatocellular carcinoma (HCC) or cholangiocarcinoma rarely justifies a liver resection. The presence of major vascular invasion or grossly positive portal lymph nodes in these patients are relative contraindications to resection, as the long-term prognosis is poor [3]. In contrast, favorable outcomes can be achieved in patients with metastatic colorectal cancer (mCRC) and multiple liver lesions, including those with lesions in both the right and left hemilivers (bilobar disease), or extrahepatic disease [4]. Nevertheless, the extrahepatic disease should be limited and resectable [5], and all the liver lesions should be amenable to treatment with resection and/or ablation.
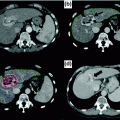
The resectability of the lesions is determined with high-quality imaging. Computed tomography (CT) scan is usually the method of choice, but magnetic resonance imaging (MRI) is emerging as a very useful imaging adjunct, especially in patients with steatosis or lesions that cannot be visualized after preoperative chemotherapy [6]. The location of the hepatic lesions and their relationship to the main hepatic vessels and the biliary tree will ultimately determine the extent of the hepatic resection and lesion resectability (Fig. 6.1). The goal of resection is to obtain negative margins, while leaving adequate functional liver with an intact hepatic arterial and portal venous inflow, venous outflow, and biliary drainage in continuity with the small bowel. For metastatic disease, the number and size of the lesions are no longer determinants of resection as long as the aforementioned requirement is met. While large lesions may involve several segments of the liver, centrally located lesions, even if small, often cannot be removed without compromising biliary drainage, vascular inflow, or the venous outflow of several liver segments. Consequently, removal of these small, central lesions may require resection of a large amount of functional liver parenchyma, thereby increasing the risk of postoperative liver failure. The adequate volume of the remnant liver is dependent on its function (see Sect. 6.3).
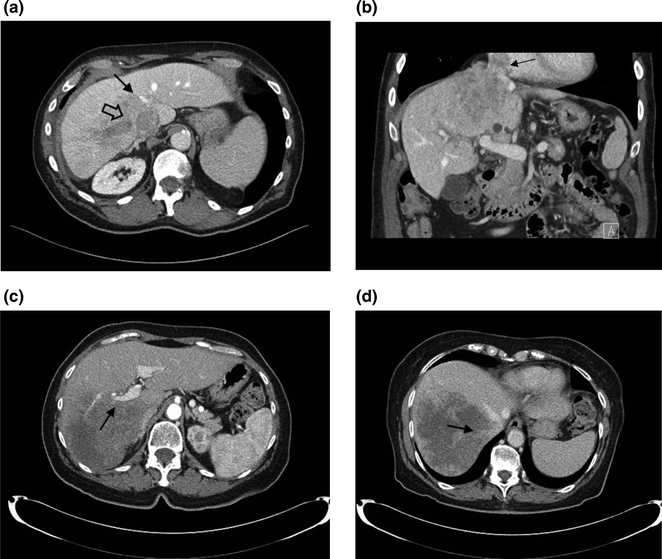

Fig. 6.1
Hepatic lesions and their relationship with hepatic vessels. a a lesion on the right liver encases the right hepatic vein (thick arrow) and abuts the middle hepatic vein (thin arrow); b coronal reconstruction shows invasion of the tumor into the IVC, extending to the right atrium (arrow); c a lesion on the right liver encases the right portal vein (arrow); and d the right hepatic vein (arrow)
The risk for postoperative liver failure and death is most pronounced in patients with chronic liver dysfunction, especially when there is evidence of cirrhosis. Even minor liver resections can result in rapid liver decompensation following surgery in these patients. Therefore, surgical resection is typically limited to cirrhotic patients with Child-Turcotte-Pugh (CTP) class A and no evidence of significant portal hypertension [7]. The presence of portal hypertension is based on a history of variceal bleed, the presence of thrombocytopenia or evidence of esophageal varices, and splenomegaly on imaging. When in doubt, the hepatic venous pressure gradient can be measured. A pressure gradient greater than or equal to 10 mmHg is associated with an increased risk of decompensation after surgery [8]. Patients with HCC and cirrhosis CTP B or C or with evidence of portal hypertension should be treated with liver transplantation or other modalities if transplantation is contraindicated.
6.3 Preoperative Assessment
Patients considered for surgical resection should have a perioperative risk assessment with clinical optimization of the patient’s medical comorbidities and functional status. Complete staging should be performed with CT of the chest and tumor markers as indicated. Positron emission tomography (PET) should be used selectively in patients with mCRC at high risk for extrahepatic metastasis, as higher rates of false negative findings limit its use in patients undergoing chemotherapy or when lesions are smaller than 1 cm. In fact, the use of PET prior to liver resection has been shown to results in changes in the surgical management of less than 10% of the patients and no differences in long-term survival [9].
The function of the liver is typically estimated through liver function tests such as total bilirubin, prothrombin time, and albumin. Imaging-based liver function tests are frequently used in Europe and Asia to determine the extension of the liver parenchyma that can be safely removed [10]. The indocyanine green (ICG) test is the most commonly used method, but new techniques such as 99mTc galactosyl and the 99mTc mebrofenin scintigraphy with single-photon emission computer tomography (SEPCT-CT) [11] and the Gd-EOB-enhanced MRI [12] have been recently introduced and may have an increasing role in the preoperative evaluation of these patients, as they add spatial information [13].
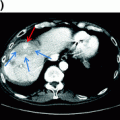
If a major liver resection is planned, the volume of the postoperative liver, or future liver remnant (FLR), is estimated. The volume of the FLR needed to minimize the chances of postoperative liver insufficiency is dependent on the liver remnant’s function. Liver dysfunction is common in patients presenting for resection of hepatic malignancies. For example, hepatocellular carcinoma and cholangiocarcinoma are often associated with chronic liver disease, and prolonged modern chemotherapy is associated with significant injury to the liver, as discussed later. For patients with no liver dysfunction, the FLR should be at least 20% of the total liver volume (i.e., one can remove up to 80% of the liver) [14]. Patients with abnormal background liver (i.e., following preoperative chemotherapy) should have a FLR of at least 30% of the total liver volume, while those with cirrhosis should have a FLR of at least 40% [15]. When questionable, formal calculation of the FLR should be performed. The volume of the expected remnant liver and the total liver volume are measured using either CT scan or MRI. The volume of the non-functional liver (parenchyma, i.e., either non-perfused or replaced by tumor) is subtracted from the total liver volume, which is especially important for patients with large lesions. Alternatively, the total liver volume can be estimated with different formulas based on the patient’s weight or body surface area [16]. Portal vein embolization should be considered for patients with insufficient FLR. Portal vein embolization involves selective occlusion of the branches of the portal vein feeding the segments planned for resection, which stimulates contralateral hypertrophy (Fig. 6.2). The FLR volume increases significantly within the first 3–4 weeks after the procedure. Liver volumetry is then repeated to assure a minimal FLR volume has been achieved. Otherwise, imaging is repeated in another 4 weeks, as liver regeneration may continue to occur, albeit at a slower rate [17]. The degree of hypertrophy of the remnant (at least 5% increase in volume or a rate of at least 2% increase per week) has been associated with decreased rates of postoperative liver insufficiency [17, 18].
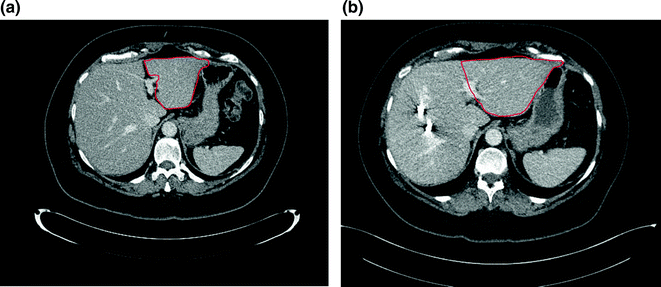

Fig. 6.2
Future liver remnant (FLR) before (a) and after portal vein embolization (b). The volume of the FLR (in red) increased by 30% four weeks after portal vein embolization
6.4 Effect of Chemotherapy and Radiation
Preoperative chemotherapy is typically administered to patients with mCRC and, at times, cholangiocarcinoma to assess tumor response, convert unresectable tumors to resectable and to address micrometastatic disease that is not seen on imaging. Nevertheless, preoperative chemotherapy has not been shown to improve survival in patients for hepatic resection for mCRC [19]. Moreover, prolonged modern chemotherapy for colorectal cancer may be associated with significant injury to the liver, with increased risk of postoperative complications following liver resection. Specifically, irinotecan-based treatment is associated with steatohepatitis, while oxaliplatin-based chemotherapy is associated with sinusoidal congestion [20]. Also, small (<2 cm) lesions may disappear on imaging with chemotherapy. If not surgically resected, these lesions will recur in up to 80% of the patients [21]. Surgeons and the medical oncologists need to work closely to determine optimal duration of preoperative chemotherapy and the timing for liver resection. In general, if preoperative chemotherapy is used, the duration should be limited to 2–3 months.
Selective internal radiation therapy (SIRT) with Yttrium-90 has been increasingly used in the treatment of liver primary and secondary malignancies, mostly in the palliative setting. Recent studies have been shown that SIRT to one hemiliver often results in significant hypertrophy of the contralateral side [22]. Such findings motivated the use of SIRT as a substitute to portal vein embolization prior to liver resection, with the advantage of providing tumor control in the involved segments while the uninvolved liver grows. Clinical experience is limited with this approach, with some reports describing increases in the technical complexity of the operation, as the liver parenchyma becomes fibrotic and inflexible [23]. There are inconsistent data on the impact of these findings in terms of perioperative outcomes [24, 25].
6.5 Types of Liver Resection
Liver resections can be categorized into anatomic and nonanatomic (wedge resections). Anatomic resections involve the resection of portal territories and include segmentectomies, sectionectomies, hemihepatectomies, and extended hepatectomies. If feasible, anatomic resections should be performed for the treatment of HCC, as HCC tends to spread via portal venous tributaries. In fact, anatomic resections have been associated with improved survival in patients with HCC in observational studies [26]. In contrast, when treating mCRC, superficial resection can be performed nonanatomically, with the goal of achieving microscopically negative margins. The width of the negative surgical margins has not been shown to be associated with increased risk of local recurrence. This strategy, referred to as parenchyma-sparing hepatectomies, is preferred over major hepatectomies for mCRC, when applicable, as these procedures are associated with decreased morbidity and increased rates of salvage ability in cases of recurrence—with no increase in recurrence rate or decrease in overall survival [27].
Liver resections are also defined based on the extension of the resection into minor and major resections. Major hepatic resection involves three or more liver segments based on Couinaud’s classification. According to the Brisbane 2000 Nomenclature of Hepatic Anatomy and Resections, proposed by the International Hepato-Pancreato-Biliary Association in an effort to standardize the terminology in the field, the anatomic division of the liver is based on the vascular watershed—the plane intersecting the gallbladder fossa and the inferior vena cava (IVC), described by Cantlie in 1897 and not seen from the surface of the liver [28]. This nomenclature rendered use of the term liver lobe obsolete, as this implies the presence of a visible anatomic demarcation (i.e., the umbilical fissure). Such principles were used to define the current terminology for liver resections (Table 6.2), which should no longer be referred to as lobectomies.
Table 6.2
Nomenclature of major hepatic resections based on the Brisbane 2000 nomenclature of hepatic anatomy and resections
Major hepatic resection | Liver segments resecteda |
|---|---|
Right hepatectomy | 5, 6, 7, 8 |
Extended right hepatectomy | 4, 5, 6, 7, 8 |
Left hepatectomy | 2, 3, 4 |
Extended left hepatectomy | 2, 3, 4, 5, 8 |
Minimally invasive (laparoscopic or robotic) liver resection can be performed in selected patients. Observational data suggest that laparoscopic liver resection is associated with decreased wound complications, intraoperative blood loss, postoperative pain, and length of stay while being associated with similar mortality, positive margin rates, and long-term outcomes in patients with mCRC or HCC [29–31]. Because laparoscopic liver resections are often applied to small, peripheral tumors, there is certainly potential for selection bias. Randomized trials comparing laparoscopic versus open liver resection are currently ongoing.
6.6 Combined Procedures
Liver resection can be done in combination with other procedures such as ablation of liver lesions or resection of the primary tumor in metastatic disease.
Liver resection can be combined with intraoperative ablation of lesions, consistent with the goal of sparing of liver parenchyma. For bilobar metastases, a major liver resection can be performed on the side with the highest disease burden, while ablation is performed on the contralateral side. These procedures may be performed at the same time if the anticipated volume of the FLR is adequate. Otherwise, separate, staged operations should be considered. Ablation can also be used intraoperatively to treat centrally located lesions that would otherwise require a major liver resection in combination with wedge or segmental resection of more superficial lesions in patients with multiple metastases. Ablation is typically thermal, using heat (radiofrequency and microwave ablation) or cold (cryoablation), with the latter rarely used nowadays. Irreversible electroporation, a newer technology based on the short-duration, high-voltage pulses that result in defects in the lipid bilayer—and, ultimately, cell necrosis—is emerging as an attractive substitute to thermal ablation, especially near major vessels, as it is not associated with deflection in the ablation zone due to heat loss near adjacent vascular structures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree