Fig. 11.1
Cumulative mortality for those patients undergoing weight loss surgery is significantly lower than a control group—patients received the customary nonsurgical treatment, ranging from lifestyle intervention and behavior medication to no treatment
Patients who are 100 pounds overweight and who have had a bariatric operation achieve superior weight loss, are healthier and live longer. It is reasonable for patients with a Body Mass Index (BMI) greater than 40 kg/m2, or those with a BMI >35 kg/m2 with a weight related co-morbidity, or those with a BMI ranging from 30-34.9 kg/m2 and either Type II Diabetes Mellitus or Dysmetabolic Syndrome X to be offered WLS, as the risk of not having an operation for exceeds the risk of undergoing an operation. The most common causes of death after bariatric surgery are from a pulmonary embolism (PE), sepsis most often due to an anastomotic leak, cardiac events such as a myocardial infarction, and respiratory failure.
Pulmonary Embolism
Obesity is a risk factor for deep venous thrombosis (DVT) and PE. The incidence of symptomatic PEs ranges from 0 to 5.4 % [10]. Fifty percent of deaths in the perioperative period are caused by PE, making it the most common cause of death. The overall rate of in-hospital venous thrombotic event (VTE) was 0.17% with the highest rate observed in an open RYGB at 0.45%. [11]. While there is no standard for VTE prophylaxis perioperatively, many surgeons will use both chemoprophylaxis and mechanical prophylaxis via sequential compression devices (SCDs). Bariatric surgery candidates who are nonambulatory, have a history of venous stasis disorders, use hormonal therapy, have obesity hypoventilation syndrome, or have pulmonary hypertension are considered an even higher risk. Chemoprophylaxis is often given to these higher-risk patients for a longer duration like 3-6 weeks post operatively. Enoxaparin for a longer duration is often given to these higher-risk patients. In 2013, the American Society for Metabolic and Bariatric Surgery (ASMBS) published an updated position statement recommending perioperative VTE prophylaxis, but did not recommend a standardized dosing protocol [10]. There is support to use a combination of chemoprophylaxis and mechanical prophylaxis (SCDs), and though enoxaparin or heparin may be used, the highest-quality data suggest the use of enoxaparin.
Use of filters is controversial as the potential complications of inferior vena cava (IVC) filters are not insignificant and insertion and removal in super-obese patients may prove to be technically challenging for an interventionalist. A prospective clinical registry involving 20 Michigan hospitals accrued 6376 patients undergoing gastric bypass surgery between 2006 and 2008 [12]. IVC filter placement and complications within 30 days of surgery were analyzed in 542 gastric bypass patients (8.5 %) who underwent preoperative IVC filter placement. In patients with IVC filters, they noticed an increased rates of postoperative VTE (1.7 vs. 0.5 % p = 0.001), death (1.0 vs. 0.2 % p = 0.01), and any complications (16.3 vs. 9.5 % p < 0.001). These complications included IVC thrombosis, filter migration, and embolization. Furthermore, the authors were unable to identify any patient subgroup for whom IVC filters were associated with improved outcomes .
Anastomotic Leaks
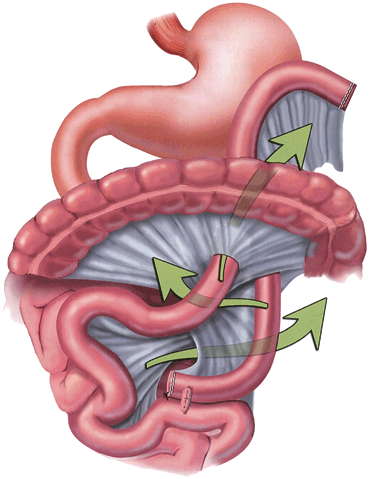
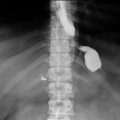
Leaks are the most dreaded complication and one of the most common causes of postoperative mortality.During the operation, surgeons will inspect all staple lines, and may also inject methytlene blue saline, perform air insufflation, or perform endoscopy to assess for a leak. After an RYGB, a leak may occur at the gastojejunostomy (GJ) (Fig. 11.2 ), jejunojejunostomy (JJ), or the staple line of the remnant stomach. A contrast swallow study might identify a leak at the GJ but miss other locations, and is unreliable for excluding a leak. Performing a computed tomography (CT) scan with oral contrast might identify free air, a fluid collection or extravasation of contrast. Early diagnosis is imperative. Tachycardia and respiratory compromise are early signs of a leak, signs that are present before hypotension, decreased urinary output, and abdominal pain. Another reported sign is a patient who has a “feeling of doom” [13]. Patients presenting postoperatively with shortness of breath are statistically more likely to have a leak than PE, and the surgeon should be promptly notified as a leak usually requires prompt return to the operating room for exploration. In the immediate postoperative period, an unexplained heart rate of greater than 120 beats/min is a leak until proven otherwise, which might require reoperation, drains, and a gastric feeding tube. Delay in treatment is a common cause of death. Re-exploration would be warranted if an MI, PE, bleeding, atelectasis, hypovolemia, and pain (all potential causes of a postoperative tachycardia) are ruled out or treated and the patient remains tachycardic. A CT scan or swallow study may not be reliable to detect a small leak, leaving re-exploration as the only option for detection.
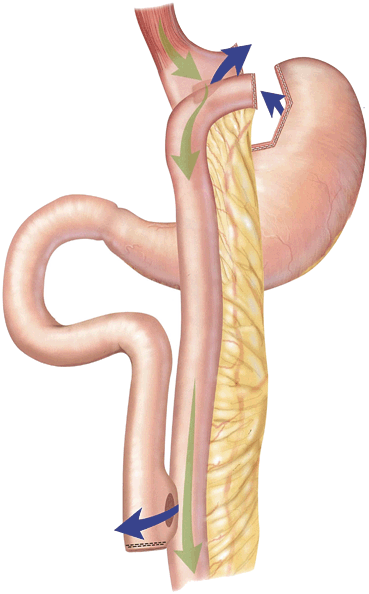

Fig. 11.2
Sites of potential leaks at the GJ, remnant stomach, and JJ; depicted by blue arrows. GJ gastojejunostomy, JJ jejunojejunostomy
Leaks and perforations can also occur with an AGB, an SG, and a BPD-DS. With an SG and BPD-DS, a leak along the resected stomach can be very problematic as the narrow sleeve is a high-pressure zone. Once a leak is present, it tends to persist. This may be the reason, along with the long staple line, that the leak rate after an SG is the highest, up to 10 % [14], double the rate of leak after RYGB [6, 15]. While drainage alone may work to close the leak, sometimes an endoscopically placed stent or clip is required. If a leak after SG is associated with a distal obstruction , conversion to gastric bypass may be required to turn a high-pressure leak into a lower-pressure leak, thus giving the patient a better chance of healing.
Leaks can occur several weeks after the operation and providers must have a high index of suspicion for them so that they can attempt to diagnose them early. It is generally safer to reoperate to rule out a leak rather than allow it to become clinically obvious. By that time, the patient may already be suffering pulmonary, cardiac, and septic complications. Unfortunately, the diagnosis and management of intra-abdominal infections are more challenging in a morbidly obese patient, so a high index of suspicion is always warranted.
Cardiac Complications
Cardiac ischemic events are the second most common cause of perioperative death in bariatric surgery patients [16] accounting for 12.5–17.6 % of cases [17]. That said, cardiac events occur in < 1 % of patients undergoing bariatric surgery [15]. Cardiovascular complications in the postoperative period decrease over time [18]. As many obese patients often have several comorbidities related to cardiac disease, a preoperative risk assessment should be considered in every patient.
Other Respiratory Complications
Bariatric surgery patients also have a spectrum of respiratory obstructive and restrictive pulmonary physiologies. Atelectasis is a common occurrence after WLS occurring in 8.4 % of patients [19]. In patients with obstructive sleep apnea (OSA), noninvasive ventilation was found to improve the lung function if started within 30 min after extubation in the recovery room [20]. Many patients seeking WLS will have OSA [21]. Patients who are untreated and chronically tired will gain weight. Furthermore, sedation and narcotics after surgery place the patient at high risk for respiratory compromise and death postoperatively. An OSA diagnosis should be actively sought prior to surgery and treated with continuous positive airway pressure (CPAP) if only to get patients comfortable using a mask they will require after the operation. WLS patients with OSA should continue to use their CPAP postoperatively, even on the first postoperative night.
Using the National Surgical Quality Improvement Program (NSQIP) database, the data for 32,889 patients between 2006 and 2008, were analyzed by Gupta et al. [22]. Postoperative pneumonia and postoperative respiratory failure accounted for mortality in 18.7 % of cases. Length of stay in hospital and 30-day mortality are increased in those patients who develop postoperative pneumonia or respiratory failure. Preoperative risk factors for postoperative pneumonia were congestive heart failure (OR 5.3) and stroke (OR 4.1), as well as bleeding disorder, age > 50, chronic obstructive pulmonary disease, type of surgery, diabetes mellitus, anesthesia time, increasing weight, and smoking. Previous percutaneous coronary intervention (OR 2.8) and dyspnea at rest (OR 2.64) were the factors most strongly associated with postoperative respiratory failure. As such, all WLS candidates with these risk factors should have pulmonary function tests to evaluate their risk of postoperative pulmonary complications.
Chronic pulmonary complications may include recurrent aspiration pneumonia secondary to chronic reflux or vomiting. This could be seen with patients with stomal obstruction or stenosis [23], especially in patients with AGBs where a recent study reports a chronic respiratory complication rate of 1.4 % [24].
Stenosis or Outlet Obstruction
Stenosis or an anastomotic stricture usually presents as vomiting, or the inability to tolerate oral intake . The stenosis may be severe enough that the patient cannot swallow his or her own secretions and saliva. This complication typically occurs in the first few months after the operation, with a median presentation at 46 days [25]. Strictures occurred significantly more frequently when a 21-mm versus a 25-mm circular stapler was used for the GJ anastomosis for an RYGB [25, 26]. The size of the stapler is one technical factor in the development of a stricture, as is tension on the anastomosis, or ischemia. Another factor is the patient and his or her ability to heal. The primary treatment for stricture is endoscopic balloon dilation, and sometimes more than one dilation may be required [25]. However, caution needs to be used with dilation as perforation or bleeding are two potential complications. Balloon dilation of the anastomosis should only be performed in experienced hands, with the bariatric surgeon who is knowledgeable and experienced in endoscopy—the best provider to treat a stricture. Repeat dilation may result in swelling of the anastomosis and make future dilations more difficult, and repeat dilations make operative intervention more likely [13]. It is recommended that within the first 3 weeks postoperatively dilation should not be performed, and instead the patient should be kept on a liquid diet, or even total parenteral nutrition if they cannot tolerate enough per os (PO) nutrition. However, if dilation is going to be performed in those few weeks a smaller balloon and lower pressure should be used [25].
Patients who have undergone an SG may also present with vomiting, dysphagia, and an inability to tolerate PO, due to narrowing and gastric outlet obstruction. This complication has been reported to occur in 0.7 % of patients [27]. The ideal bougie size for construction of the sleeve has yet to be determined, currently ranging from 32 to 40 fr, and many surgeons currently use a 36-fr bougie [13]. Too small of a size may lead to narrowing and obstruction, but too large of a size may result in less weight loss . Treatment for these patients is also endoscopic dilation, unless the segment of narrowing is too long, in which case they may need repeat surgery and conversion to an RYGB.
Patients who have had an AGB may present with acute obstruction in the early period. This is usually due to bleeding underneath the band, or failure to remove the esophageal fat pad. In general, this can be managed by waiting for the edema to resolve after a few days or by surgical intervention to remove the hematoma or fat pad [28].
Late Postoperative Complications
Failure to Achieve Weight Loss or Weight Regain
Most patients lose weight after weight loss operations. In general, AGB patients can lose 30–70 % excess weight loss (EWL) or 30–70 pounds of every hundred pounds above the ideal body weight (IBW). O’Brien et al. [29] showed a 47 % EWL even out to 15 years after AGB (Fig. 11.3 ). After SG, patients typically lose more than 60 % EWL [30], after RYGB 60–70 % EWL [7, 31], and even more after BPD-DS. While there are metabolic, social, and behavioral reasons for failure to lose weight or to have weight regain, technical complications need to be considered.
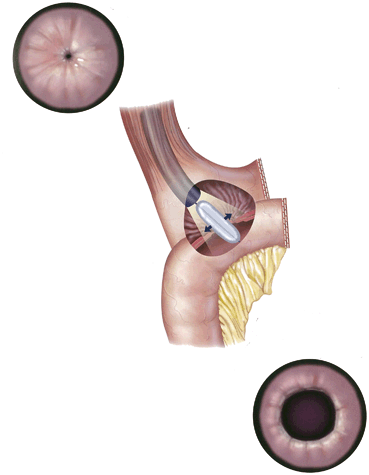

Fig. 11.3
Average weight loss over 15 years in surgery subgroups and controls not obtaining (no prof) or obtaining (prof) professional help
After AGB, if the patient is eating high-calorie liquid foods such as ice cream and alcohol, they will not have appropriate postoperative weight loss, and may even continue to gain weight. These foods pass the band easily and can counter any weight loss efforts made by the patient. Alternatively, the band may not be achieving adequate restriction because it is not properly adjusted or because there is a leak in the tubing. If the band is not adequately restrictive then satiety is not achieved and larger portions of food are consumed. Using a Huber needle, the port can be accessed and the physician can determine the volume of fluid within the system and instill additional fluid into the band. If less fluid is withdrawn than expected, a leak may be present and the port or the band may need to be replaced. A leak may be due to fractured tubing, or at other times a slow leak can occur from a previous needle puncture. Overall, the band has a reoperation rate of up to 40 % for complications such as leaks, band slips, band erosions, or failure to lose or sustain weight loss [29].
Even after a SG, a RYGB or a BPD-DS, patients may regain weight. Types of food eaten, amount of alcohol consumed, increasing portions or frequency of meals, decreasing exercise, and stress as it relates to eating and exercise should all be assessed by the PCP. It is important to encourage patients to have lifelong follow-up, and it is helpful for the patient to be monitored by registered dietitians, psychologists, bariatricians, and the operating surgeon. Having this lifelong follow-up can help prevent weight recidivism and also assist with identification of postoperative complications. The lowest weight on average is obtained 18 months after RYGB with weight regain becoming significant within 4 years after surgery [31]. A contrast study may be helpful to exclude a fistula from the pouch to the remnant stomach after RYGB or vertical banded gastroplasty, as a technical reason why patients may be able to eat much larger quantities of food than immediately after surgery. Of course, the stomach can also be stretched over time, but efforts to downsize the pouch have had poor results at achieving weight loss after postoperative weight gain. It remains to be seen if the SG results in sustained weight loss for more than 10 years. The overwhelming reason for weight regain after any operation is a failure to consistently live a healthy lifestyle that is conducive to sustained weight loss.
Too Much Weight Loss
This, too, can happen albeit infrequently. While anorexia and bulimic behaviors may be the cause, technical complications can also cause EWL. It can be very helpful to have a dietitian review the patient’s food logs to help ensure they are getting adequate calorie intake. An AGB that is too tight and too restrictive may be easily corrected by withdrawing fluid from the band. Similarly, stenosis or obstruction that can occur after SG, RYGB, and BPD-DS can also cause excessive weight loss . An upper gastrointestinal (GI) series and a small bowel follow through or CT scan can also inform the surgeon if there is a stenosis or obstruction . Typically, weight loss will stop after a couple years as patients become accustomed to the restriction and instead begin to eat more often. The malabsorption component may also be overcome with time, though it is uncertain how much remains long term.
Persistent Vomiting
Vomiting can occur from eating too fast or too large of a portion, and all patients should be instructed to measure their portions, eat small bites, chew well, and eat slowly. If vomiting persists, something is wrong, and if left unaddressed can cause significant nutritional deficiencies. After an AGB, the band may be too tight and simply need to be adjusted. If this does not resolve the patient’s nausea and vomiting, then the physician needs to consider that there is a prolapse, whereby the stomach below the band slides above the band, often obstructing the stoma and sometimes becoming a surgical emergency if the gastric blood supply is compromised. This can be diagnosed with an upper GI series or a CT scan and if it occurs, requires an operation to remove or replace the band.
After an SG, vomiting might occur if the sleeve has strictured, twisted, or was too narrow to begin with. This can be easily assessed by an upper GI series study by noting slow movement of contrast, or failure of contrast to progress. This can be treated with stenting or balloon dilation (Fig. 11.4 ) but may also require a revision to an RYGB.
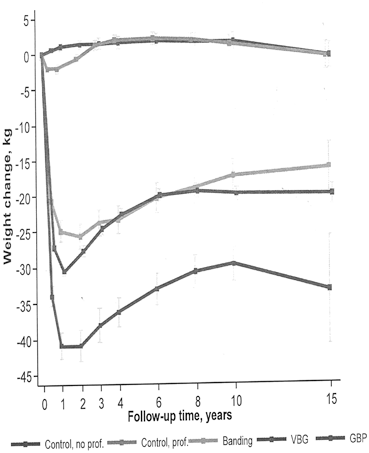

Fig. 11.4
Stenosis of the GJ, before and after treatment with balloon dilation. GJ gastojejunostomy
After an RYGB or a BPD-DS, vomiting may occur if there is a stenosis of the GJ anastomosis. Often endoscopic dilation(s) can manage this complication, as mentioned earlier. The patient may first present with difficulty swallowing solid foods and then not be able to tolerate liquids as the anastomosis further narrows. Persistent vomiting again may rapidly cause a thiamine deficiency and requires supplementation. Thiamine levels should be checked and a thorough neurologic exam should be done if the patient endorses continued emesis. Other causes of vomiting may be a small bowel obstruction, from either adhesions or a hernia.
Abdominal Pain
Abdominal pain in a bariatric patient needs to be taken seriously, as they have not only the usual adult causes of abdominal pain but the cause of their abdominal pain may be due to a specific complication of their surgery as well. Abdominal pain may be difficult at times to diagnose in a bariatric patient given their rerouted anatomy, as well as the fact that these patients often have vague symptoms and an unreliable physical examination given their habitus. Unfortunately, sometimes bariatric patients continue to have abdominal pain of unknown origin, even after extensive workup for defined causes.
Hernias
Hernias can occur at sites of previous incisions. Small port-site hernias may be difficult to diagnose without a CT scan and can cause significant pain for a patient. Hernias are more common with larger incisions such as laparotomy (open operation) or single incision laparoscopic surgery (SILS) whose smaller incisions can be up to 3–4 cm. A midline laparotomy in an obese patient has a 20–41 % chance of hernia development [32]. Port-site hernias should be repaired promptly given that the small size makes incarceration or strangulation more likely, whereas large incisional hernias may be best to delay repair until weight loss is achieved. This will lower the rate of a recurrent hernia. Mesh should be used during a definitive repair as it is more durable than sutures alone.
Another type of a hernia that often presents as abdominal pain is an internal hernia. When a retrocolic Roux limb is created, the Roux limb travels behind the colon up to the stomach pouch; and consequently, three potential spaces are created. They are the opening of the transverse mesocolon that allows passage of the Roux limb, the space beneath the Roux limb but above the transverse mesocolon, also known as the Peterson’s Defect, and the mesenteric defect of the J-J anastomosis.These defects must be closed with sutures, otherwise the openings in the mesentery allow the small bowel to herniate through the defect and cause a small bowel obstruction and pain. Additionally, after significant weight loss, the sutures that were used to close the potential hernia defects may loosen causing an internal hernia.
Patients may present with intermittent severe mid-abdominal pain. A CT scan should be obtained and may identify a “swirl sign.” However, the hernia may not be visualized with a CT scan, especially if the hernia is self-reduced at the time of imaging. If after an extensive workup there is no other cause of abdominal pain, a surgeon may elect to explore in the operating room, as this is the definite way to determine if there is a hernia. As with any hernia, if the small bowel blood supply is compromised, the bowel can become ischemic and require emergent resection. Thus, an internal hernia can become a surgical emergency.
Marginal Ulcers
Another cause of abdominal pain may be an ulcer. Marginal ulcers usually are distal to the GJ anastomosis and result from gastric acid irritating the mucosa of the jejunum. Some surgeons will test all patients for Helicobacter pylori prior to surgery, and treat if positive. Schirmer et al. [33] found that 30.1 % of patients tested had H. pylori. They were treated and subsequently had a lower incidence of marginal ulcers, 2.4 versus 6.8 %. Ulcers can present with not only pain but also nausea, bleeding, or even perforation. Smoking, NSAIDs, and foreign bodies, such as sutures or staples, have both been linked to development of marginal ulcers. Patients can also have ulcers appear in their remnant stomach and duodenum. They may also present with pain, bleeding, or even perforation. At most institutions, patients are put on an H2-receptor blocker or proton-pump inhibitor postoperatively for a few weeks to months after an RYGB.
Obstruction
Obstruction has been said to occur on average from 0.3 to 7 months postoperatively, but Capella et al. [34] saw the majority of obstructions occurring between 6 and 24 months, and they may continue to occur years after surgery. Early obstructions occur within 6 weeks after the operation and are likely due to technical issues resulting in the internal hernias described above (Fig. 11.5 ). Adhesions are another potential cause of a small bowel obstruction. The incidence of obstruction after laparoscopic RYGB ranges anywhere from 0.2 to 9.7 % [13, 34]. Almost 20 % of patients who presented with obstruction had a second obstruction [34]. Patients who appear to have an obstruction caused by an adhesion may be given a trial of nasogastric decompression, but the nasogastric tube placement needs to be performed by an experienced provider and there should be consideration of placing it under fluoroscopic guidance due to the risk of perforation of the small gastric pouch which could be catastrophic [13]. Intussusception, though a rare cause of small bowel obstruction , may also present as with pain, nausea, and vomiting. Intussusception accounts for approximately 1 % of cases of small bowel obstruction [35]. This complication may develop several years after a RYGB and those patients who have lost more than 90 % EBW are at the highest risk [13, 35]. Intussusception that involves the JJ anastomosis should have the JJ anastomosis revised surgically. Although rare, intussusception needs to be considered given that it can lead to bowel ischemia and necrosis, and CT scan only has an accuracy of approximately 80 % in identifying this complication [36] and a normal scan does not rule out this diagnosis, as it also does not rule out an internal hernia .