Fig. 8.1
The status of lymph node metastases according to the location of the primary tumor as reported by Ando et al. [7]
The concept of extensive three-field lymph node dissection including the dissection of cervical, mediastinal, and abdominal lymph nodes for surgically curable esophageal cancer located in the middle or upper thoracic esophagus was developed in Japan in the 1980s [6]. Although the effectiveness of extended lymphadenectomy for esophageal cancer has not yet been proven by randomized prospective studies [8, 9], many Japanese surgeons and some Western surgeons have reported the importance of radical lymph node dissection for locoregional control of esophageal cancer [3, 6–13]. This procedure has amassed little interest in Western countries. For the most part, the majority of Western esophageal surgeons have removed the readily accessible regional lymph nodes at the time of esophagectomy for the purpose of staging rather than with any expectation of improving survival [14]. A possible biological difference in these tumors in these respective countries has been suggested as a reason for the differences in the procedure of esophagectomy between Japan and Western countries. Our standard surgery for thoracic esophagus SCC is introduced in this chapter.
8.2 Surgery for SCC of Thoracic Esophagus
8.2.1 Esophagectomy
Curative resection of the primary lesion includes the removal of the gross lesion itself as well as possible concomitant spread of esophageal carcinoma. Because sufficient dissection of mediastinal lymph node is necessary, right thoracotomy and lymph node dissection plus total extirpation of the thoracoabdominal esophagus are generally performed.
8.2.2 Regional Extent of Lymphadenectomy
The distribution and incidence of lymph node metastasis might vary according to the location, size, and depth of tumor invasion. Therefore, preoperative evaluation using computed tomography, ultrasonography, magnetic resonance imaging, or positron emission tomography for each patient is important for determining the extent of the lymph node dissection. The naming and numbers of lymph nodes defined according to the location of lymph nodes [15] are shown in Fig. 8.2.
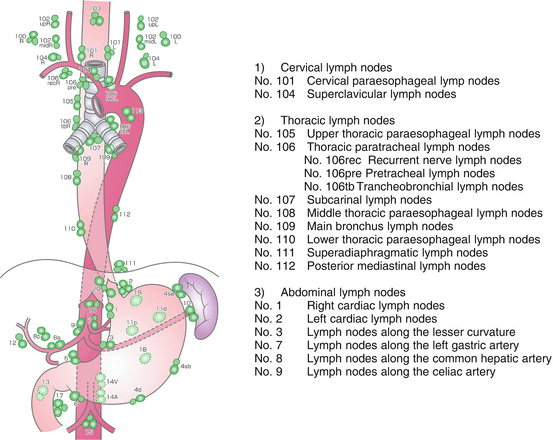

Fig. 8.2
The naming and numbers of lymph nodes defined according to the location of the nodes
8.2.2.1 Upper Thoracic Esophageal Carcinoma
In cases of upper thoracic esophageal carcinoma, lymph node metastasis occurs mainly in the cervical and upper mediastinal region. Although metastasis to lower mediastinal or abdominal lymph nodes is less frequent, dissection usually covers all three regions.
8.2.2.2 Middle Thoracic Esophageal Carcinoma
In cases of middle thoracic esophageal carcinoma, lymph node metastasis occurs mainly in cervical, the upper, middle, and lower mediastinal, and abdominal regions. The cervical approach is necessary to achieve a secure dissection of the cervical lymph nodes, including those of the supraclavicular region.
8.2.2.3 Lower Thoracic Esophageal Carcinoma
In cases of lower thoracic esophageal carcinoma, lymph node metastasis occurs mainly in the mediastinal and abdominal regions, but metastasis to the cervical lymph nodes might also occur at a lower frequency. This dissection approach is controversial, and some advocate the cervical approach; however, others regard the thoracic approach as the most adequate procedure.
8.3 Surgical Procedure
8.3.1 Surgical Approach
The open approaches used for esophageal resection include transhiatal approach, right transthoracic approach, left transthoracic approach, and left thoracoabdominal approach. The choice of approaches depends on various factors such as the location of the tumor, the general condition of the patient, and the choice of conduit for esophageal reconstruction. Because of the necessity for sufficient dissection of the mediastinal lymph nodes, the standard approach for thoracic esophageal SCC is right thoracotomy and mediastinal lymph node dissection plus the total extirpation of the thoracoabdominal esophagus.
8.3.2 Upper Mediastinal Procedure
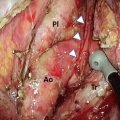
After the azygous arch was divided, the posterior side of the right upper mediastinal pleura was incised along the posterior edge of the esophagus up to the right subclavian artery. The right bronchial artery was carefully isolated and preserved for the open esophagectomy. The dorsal and left sides of the upper esophagus were dissected from the left pleura. The anterior side of the right upper mediastinal pleura was incised along the right vagal nerve up to the right subclavian artery. The right recurrent laryngeal nerve was identified at the caudal end of the right subclavian artery, and the lymph nodes around the right recurrent laryngeal nerve were carefully dissected to prevent nerve injury (Fig. 8.3). The anterior part of the upper esophagus was circumferentially dissected along with the surrounding nodes. By shifting the taped esophagus posteriorly and retracting the trachea anteriorly, it was possible to approach the left anterior side of trachea. The lymph nodes around the left recurrent laryngeal nerve were dissected from the aortic arch to the cervical area. The left subclavian artery was exposed to dissect the left recurrent laryngeal lymph nodes. During dissection of the left tracheobronchial lymph nodes, the left recurrent laryngeal nerve and left bronchial artery were preserved on the face of the trunk of the left pulmonary artery between the aortic arch and the left main bronchus (Fig. 8.4).
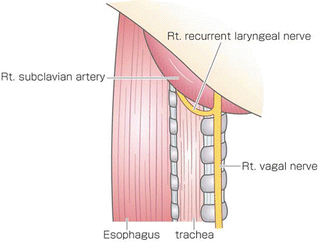
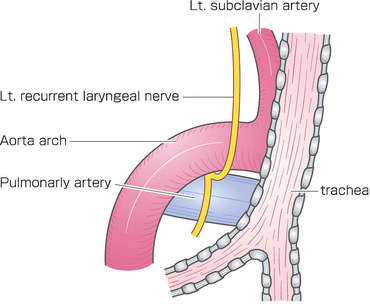

Fig. 8.3
The right recurrent laryngeal nerve was identified at the caudal end of the right subclavian artery, and lymph nodes around the right recurrent laryngeal nerve were dissected

Fig. 8.4
The left subclavian artery was exposed to dissect the left recurrent laryngeal lymph nodes. The trunk of the left pulmonary artery between the aortic arch and the left main bronchus was exposed to dissect the left tracheobronchial lymph nodes
8.3.3 Middle and Lower Mediastinal Procedure
The middle and lower mediastinal pleura tissue was incised along the anterior edge of the vertebrae to the hiatus. The posterior side of the middle to lower esophagus was dissected to expose the aortic arch and the descending aorta (Fig. 8.5). The thoracic duct was ligated and divided behind the lower esophagus and resected combined with the esophagus. The esophagus was divided using a linear stapler above the primary tumor, and the proximal stump of the resected esophagus and surrounding tissue were dissected up to the hiatus. The subcarinal nodes were separately resected (Fig. 8.6). Esophageal mobilization and mediastinal lymphadenectomy were thus completed.
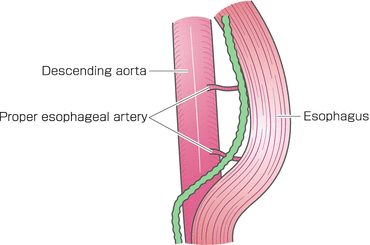
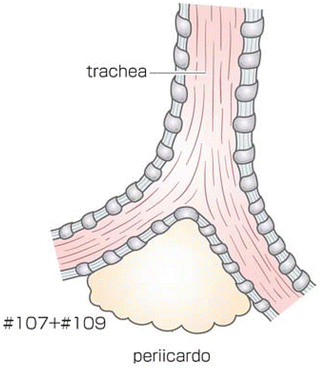

Fig. 8.5
The posterior side of the middle to lower esophagus was dissected to expose the descending aorta. The thoracic duct was ligated and divided behind the lower esophagus and resected together with the esophagus

Fig. 8.6
The subcarinal nodes were separately dissected
8.3.4 Abdominal Procedures
The greater omentum was divided 4–5 cm from the arcade of the gastroepiploic vessels. The left gastroepiploic and short gastric vessels were divided along the splenic hilum. The lesser omentum was opened, and the right gastric vessels were preserved. The distal esophagus was dissected and mobilized. The distal stump of the esophagus and the dissected mediastinal tissue were then extracted from the thorax to the abdomen. The lymph nodes around the celiac artery were dissected up to the hiatus. The stomach was divided from the lesser curvature to the fornix using linear staplers. Thus, gastric conduit formation and abdominal lymphadenectomy were completed.
8.4 Mortality and Morbidity After Esophagectomy
Transthoracic esophagectomy is one of the most invasive surgeries. Patients have the potential for respiratory, cardiovascular, and liver complications. Despite substantial advances in preoperative risk evaluation, improved operative techniques, and perioperative management, the risk of morbidity and mortality for esophagectomy remains high.
8.4.1 Mortality
Mortality has clearly linked to surgical volume. Metzer et al. performed a meta-analysis of 13 studies evaluating the impact of surgical volume on mortality after esophagectomy [16]. They showed a clear reduction in the postoperative mortality with an increasing volume of cases each year. The main reason for this phenomenon might be that the postoperative complication rates were lower in high-volume hospitals and that the management of complications was more successful. They concluded that only with the experience of >20 esophagectomies per year could a significant reduction of the mortality, which has decreased to 4.9 %, be achieved and that surgery for esophageal carcinoma was a task for high-volume hospitals. Rodgers et al. identified surgical volume as a significant predictor of mortality in a retrospective review of the Nationwide Inpatient Sample database, which included 3,243 esophagectomies [17]. Independent risks for mortality included comorbidity, age (65 years), female sex, race, and surgical volume. The mortality rates after esophagectomy have been decreasing in Japan. The 30-day mortality rate was 6.8 % during the period from 1979 to 1982, 3.0 % during the period from 1988 to 1994, and 1.0 % in 2006 [18–20] from the data of the comprehensive registry of esophageal cancer in Japan. These mortality rates after esophagectomy were lower than those reported in other countries in the recent literature. Fujita et al. showed that the 30-day and the in-hospital mortality rates in low-volume hospitals (less than five esophagectomies per year) in Japan were triple those in the high-volume hospitals (>40 esophagectomies per year) from the data from 31,380 esophagectomies that were registered from 709 institutes during the period from 2001 to 2006 in Japan [21].
8.4.2 Morbidity
8.4.2.1 Pulmonary Complications
Pulmonary complications are the most frequent complication after esophagectomy and have been implicated in nearly two-thirds of postoperative mortalities [22]. The incidence of pneumonia has been directly linked to technical complications associated with the surgical procedure [23]. The incidence of pneumonia is reported to be higher in transthoracic esophagectomy compared with THE [24] and minimally invasive esophagectomy [25].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree