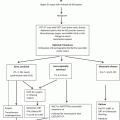
Fig. 9.1
Placement of thoracic ports. Five ports with a small thoracotomy (4–5 cm) were introduced onto the thoracic wall. ICS intercostal space, A anterior axillary line, M middle axillary line, P posterior axillary line
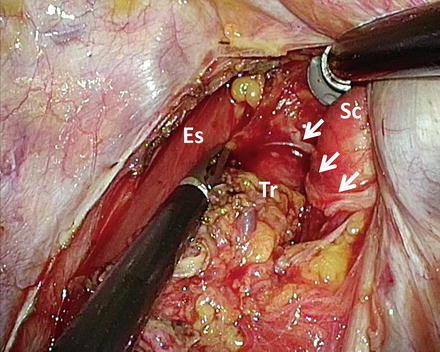
Fig. 9.2
Thoracoscopic lymphadenectomy along the right recurrent laryngeal nerve. Arrows, the right recurrent laryngeal nerve; Es esophagus, Tr trachea, Sc right subclavian artery
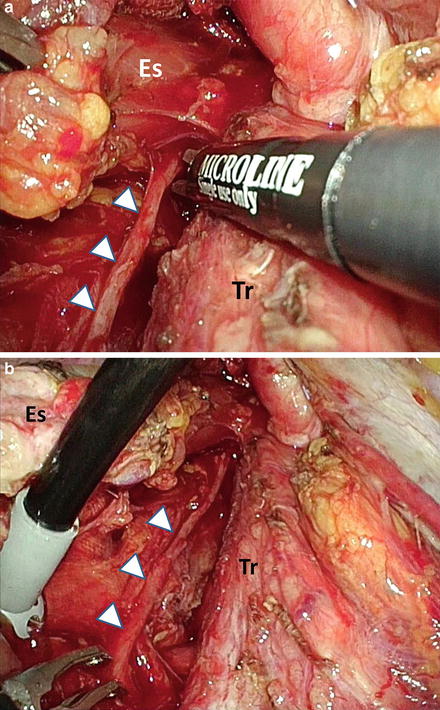
Fig. 9.3
Thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve. (a) Magnified view. (b) Overview. Arrowheads, the left recurrent laryngeal nerve; Es esophagus, Tr trachea
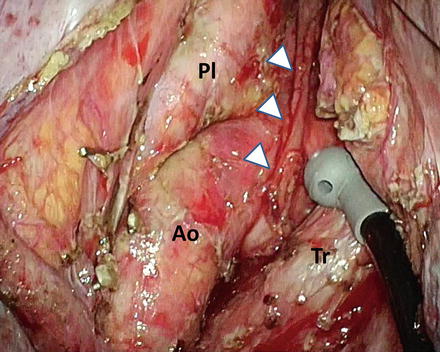
Fig. 9.4
Left upper mediastinal area after precise lymphadenectomy. Arrowheads, the left recurrent laryngeal nerve; Ao aortic arch, Tr trachea, Pl left mediastinal pleura
Subsequently, the operating table is rotated so that the patient is in the prone position, and a 7-mmHg CO2 pneumothorax is induced using a minithoracotomy lid. The mediastinal pleura is incised along the anterior edge of the vertebrae to the hiatus, and the posterior side of the middle to lower esophagus is dissected to expose the aortic arch and descending aorta. The thoracic duct is clipped behind the lower esophagus and resected together with the esophagus. The mediastinal pleura anterior to the esophagus is then incised. The esophagus is divided using a linear stapler above the primary tumor, and the caudal stump of the esophagus and surrounding tissue are dissected up to the hiatus. The subcarinal nodes are separately resected. Esophageal mobilization and mediastinal lymphadenectomy are thus completed.
The abdominal procedures are performed through an upper midline abdominal incision or by hand-assisted laparoscopic surgery (HALS). HALS procedures are performed through a transverse minilaparotomy (7 cm) in the right upper quadrant, with one port below the navel and two ports in the left abdomen.
The greater omentum, short gastric vessels, and lesser omentum are divided while avoiding injury to the right gastroepiploic and right gastric vessels under an 10-mmHg pneumoperitoneum. The distal esophagus is dissected and mobilized. The fat tissue over the left gastric artery is dissected, and the artery is divided. The distal stump of the esophagus and the dissected mediastinal tissue are then extracted from the thorax to the abdomen. The stomach is then divided from the lesser curvature to the fornix using linear staplers. Thus, gastric conduit formation and abdominal lymphadenectomy are completed.
Esophagogastrostomy is performed in the neck or thorax [26, 27]. In patients with cervical anastomoses, the gastric conduit is pulled up to the neck through the posterior mediastinal route. The cervical esophagus and gastric conduit are then anastomosed using a circular stapler. If the gastric conduit is not of sufficient length for mechanical anastomosis, the anastomosis is hand sewn. In patients with intrathoracic anastomoses, esophagogastrostomy is performed using a circular stapler at the level of the thorax in the upper posterior mediastinum through a minithoracotomy [26].
9.3 Short- and Long-Term Outcomes of MIE
9.3.1 Short-Term Outcomes of VATS Esophagectomy
To date, a number of single-institution studies have demonstrated acceptable short-term outcomes of VATS esophagectomy for thoracic esophageal cancer in terms of operating time, blood loss, and postoperative complications; these outcomes are comparable with those of conventional OE [13, 22]. Many studies have reported that the operating time of VATS esophagectomy was relatively longer than that of OE, but blood loss was markedly lesser than that of OE. Conversion of VATS esophagectomy to OE because of several reasons, such as adhesion and bulky tumor, was reported in 0–20 % of cases in single-institution studies [13]. Of note, massive active bleeding and bronchial injury were also reported as major intraoperative complications of VATS esophagectomy [13].
With regard to the number of retrieved mediastinal and/or total lymph nodes, most studies have demonstrated that VATS esophagectomy is almost equivalent to OE (Table 9.1) [38]. In terms of postoperative complications, the effect of VATS esophagectomy in reducing respiratory complications such as pneumonia remains controversial, but several studies demonstrated that the incidence of respiratory complications was significantly lower with VATS esophagectomy than with OE (Table 9.1). On the other hand, the incidence of anastomotic leak and RLN palsy with VATS esophagectomy is almost equivalent to that with OE [5]. Mamidanna et al. reported on the largest series (n = 7,502) that compared MIE with OE, using a nationwide database from 2005 to 2010 in the United Kingdom [39]. As results, there were no reports of marked differences in the overall medical morbidity (38.0 vs 39.2 %) and 30-day mortality (4.3 vs 4.0 %) between the OE and MIE groups, respectively. Furthermore, there were no marked differences in respiratory complications between the OE and MIE groups (31.4 vs 30.0 %). Of note, the reintervention rate as a result of surgical complications was significantly higher in the MIE group than in the OE group (21.0 vs 17.6 %). They concluded that the study confirmed the safety of MIE but MIE was associated with higher reintervention rates because of surgical complications, and there were no marked benefits demonstrated in the overall morbidity and mortality.
Table 9.1
Representative results of comparison between minimally invasive esophagectomy and conventional open esophagectomy for esophageal squamous cell carcinoma
Ref. # | Author (year) | # cases | SCC (%) | Operative time (min) | p value | Blood loss (ml) | p value | # Retrieved lymph nodes | p value | Respiratory complications (%) | p value | Anastomotic leak (%) | p value | In-hospital mortality (%) | p value | Hospital stay (day) | p value | Survival | p value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
[14] | Osugi (2003) | VATS 77 | 77 (100) | 227 | 0.031 | 284 | NS | 34 | NS | 15.6 | NS | 1.3 | NS | 0.0 | NS | NA | 55 % (5yOS) | NS | |
OE 72 | 72 (100) | 186 | 310 | 33 | 19.4 | 2.8 | 0.0 | 57 % (5yOS) | |||||||||||
[35] | Shiraishi (2006) | tMIE 78 | 144/153 | 426 | 0.01 | 670 | NS | NA | 20.5 | NS | 11.5 | 0.005 | 2.6 | 0.003 | NA | NA | |||
VATS 38 | (94) | 461 | 640 | 23.7 | 10.5 | 10.5 | |||||||||||||
OE 37 | 487 | 883 | 32.4 | 24.3 | 13.5 | ||||||||||||||
[36] | Gao (2011) | MIE 96 | 90 (94) | 330 | <0.01 | 347 | <0.01 | 18 | NS | 13.5 | NS | 7.3 | NS | 30-day mortality (%) 2.1 | NS | 13 | <0.01 | NA | |
OE 78 | 72 (92) | 284 | 519 | 18 | 14.1 | 7.7 | 3.8 | 18 | |||||||||||
[37] | Kinjo (2012) | tMIE 72 | 71 (99) | 308 | <0.001 | 320 | <0.001 | 28 | 0.002 | 13 | 0.001 | 4 | NA | 30-day mortality (%) 0.0 | NS | 23 | <0.001 | 72 % (2yDFS) | NS |
hMIE 34 | 31 (91) | 264 | 536 | 24 | 38 | 24 | 0.0 | 32 | 58 % (2yDFS) | ||||||||||
OE 79 | 71 (90) | 268 | 680 | 18 | 39 | 17 | 0.0 | 53 | 58 % (2yDFS) | ||||||||||
[11] | TIME trial (2012) (Randomized controlled trial) | MIE 59 | 24 (41) | 329 | 0.002 | 200 | <0.001
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|