Surgical resection remains the only potentially curative therapy for pancreatic cancer, despite a high rate of systemic recurrence. Because of local invasion or distant spread, a minority of patients presenting with pancreatic cancer are candidates for surgery. Although perioperative mortality is low in high-volume settings, pancreatic surgery remains associated with considerable morbidity. Minimally invasive and robotic surgical techniques are increasingly used for pancreatic resection, although not always applicable to all patients. Strategies to extend the benefits of margin-negative surgical resection to more patients include surgery with vascular resection and reconstruction for locally invasive tumors, and resection after neoadjuvant therapy.
Key points
- •
Surgical resection with negative margins offers the potential for cure for pancreatic cancer, although rates of local and systemic recurrence are high.
- •
Appropriate use of imaging and endoscopic techniques can determine resectability with high reliability.
- •
Surgery in high-volume centers can be performed with a perioperative mortality of 1% to 3% and 5-year survival approaching 20%, although surgical morbidity remains high.
Introduction
Pancreatic cancer is widely recognized as one of the most aggressive solid tumors and one of the most frequent causes of tumor-associated death in Western society. Surgical extirpation as part of a multimodality treatment course remains the only potentially curative therapy, although median survival is still only approximately 24 months in this select population. Furthermore, only a minority of patients presenting with pancreatic cancer are candidates for surgical therapy due to the presence of either distant metastases or locally invasive disease.
Early skepticism for pancreatic surgery is seen in Moynihan’s 1906 edition of “Abdominal Operations” in which he states that “the treatment of malignant disease of the pancreas by the surgeon can hardly be said to exist…the mechanical difficulties of the operation are well-nigh insuperable, and that if boldness and good fortune are the operator’s gifts, the result to the patient hardly justifies the means.” Whipple’s 1935 publication describing surgical management of ampullary malignancy in 80 patients resulted in his name being commonly used to describe pancreaticoduodenectomy, although the anatomic and physiologic barriers to safe pancreatic surgery were not realized until late in the twentieth century. Even in the mid 1960s, surgical morbidity of more than 60% and mortality of more than 25% persisted.
Recent decades of progress in surgical and perioperative care have seen a decrease in perioperative morbidity to less than 35% and perioperative mortality to less than 2%.
Introduction
Pancreatic cancer is widely recognized as one of the most aggressive solid tumors and one of the most frequent causes of tumor-associated death in Western society. Surgical extirpation as part of a multimodality treatment course remains the only potentially curative therapy, although median survival is still only approximately 24 months in this select population. Furthermore, only a minority of patients presenting with pancreatic cancer are candidates for surgical therapy due to the presence of either distant metastases or locally invasive disease.
Early skepticism for pancreatic surgery is seen in Moynihan’s 1906 edition of “Abdominal Operations” in which he states that “the treatment of malignant disease of the pancreas by the surgeon can hardly be said to exist…the mechanical difficulties of the operation are well-nigh insuperable, and that if boldness and good fortune are the operator’s gifts, the result to the patient hardly justifies the means.” Whipple’s 1935 publication describing surgical management of ampullary malignancy in 80 patients resulted in his name being commonly used to describe pancreaticoduodenectomy, although the anatomic and physiologic barriers to safe pancreatic surgery were not realized until late in the twentieth century. Even in the mid 1960s, surgical morbidity of more than 60% and mortality of more than 25% persisted.
Recent decades of progress in surgical and perioperative care have seen a decrease in perioperative morbidity to less than 35% and perioperative mortality to less than 2%.
Indications/Contraindications
Although surgical resection is the only potentially curative treatment for pancreatic cancer, benefit from surgical resection is limited to the subgroup of patients with localized disease in whom resection with negative surgical margins can be obtained. Patients with localized tumors are described as either “resectable,” “unresectable,” or “borderline resectable,” largely based on involvement of local vasculature. With in-depth assessment of local and distant spread, at most 20% of patients are considered clearly candidates for up-front surgical resection. See later in this article.
Thorough preoperative evaluation is essential before proceeding to surgical resection for pancreatic malignancy. Cross-sectional imaging and endoscopic techniques are used to assess involvement of nearby structures, and endoscopic retrograde cholangiopancreatography (ERCP) with biliary stenting may be required in the setting of preoperative biliary obstruction.
Imaging
Imaging studies must specifically address several pertinent points when considering patients candidates for surgical resection:
- •
Superior mesenteric vein/portal vein (SMV/PV) involvement
- •
Superior mesenteric artery involvement
- •
Celiac axis involvement
- •
Common/proper hepatic artery involvement
- •
Anatomic variants: origin of right and left hepatic artery, origin of common hepatic artery, presence of accessory hepatic arteries
- •
Regional and distant lymphadenopathy
- •
Local/regional invasion (inferior vena cava, left renal vein, left adrenal)
- •
Distant metastases (liver or lung)
Multiphase contrast-enhanced computed tomography (CT) timed to visualize both arterial and venous phases is generally the first preferred imaging method. The lack of local invasion or distant metastases can be predicted in most patients by using CT alone, particularly with multidetector helical CT scanners. Contrast-enhanced MRI is often used interchangeably with multiphase CT scan for preoperative imaging. MRI has the advantage of allowing magnetic resonance (MR) cholangiopancreatography, which offers superior visualization of the biliary or pancreatic duct. Published data do not convincingly demonstrate the superiority of either MR or CT as a cross-sectional imaging modality for detecting vascular involvement and local invasion. Although PET-CT has the potential to detect occult metastases, it has a limited role in staging or pancreatic cancer. Sensitivity for disease is reported between 46% and 71%, with sensitivity between 63% and 100%.
Endoscopic Evaluation
Endoscopic ultrasound (EUS) is useful for assessing small lesions and performing biopsy via fine-needle aspiration if pathology is required before surgical or medical therapy. EUS is useful in evaluation of resectability of pancreatic neoplasms, particularly involvement of the SMV/PV axis. EUS is particularly valuable with lesions smaller than 2 cm that may be particularly difficult to diagnose on cross-sectional imaging. Although tissue biopsy may not be necessary in a patient in whom surgery is planned for a pancreatic mass, tissue biopsy is particularly important in cases of greater diagnostic uncertainty or if definitive or neoadjuvant chemotherapy is planned. Sensitivity of EUS is reported to be between 75% and 90%, with specificity of approximately 100% for pancreatic head tumors. In a systematic review of more than 50 series of EUS for pancreatic neoplasms, the negative predictive value was only between 60% and 70%; thus, a negative biopsy must be interpreted within the appropriate clinical context and repeat biopsies may be needed if tissue diagnosis is essential to the treatment plan or clinical protocol.
ERCP is potentially useful for tumors of the pancreatic head. Cytology specimens via intraductal brushing can be of diagnostic use, although sensitivity is less than 50% for pancreatic cancer. ERCP also provides the ability to relieve biliary obstruction. Routine biliary stent drainage is potentially useful in patients with biliary obstruction in those undergoing neoadjuvant chemotherapy or chemoradiotherapy. Otherwise, ERCP with biliary stenting is not used unless required for decompression of a severely obstructed biliary system. Obstructive jaundice due to a periampullary mass can be associated with cholangitis impaired hepatic function, and altered coagulation due to decreased vitamin K absorption. Patients with bilirubin higher than 10 to 20 mg/dL or jaundice for more than 3 weeks are at particular risk for postoperative complications. However, stent-related infections are associated with increased infectious complications after pancreatic surgery. Randomized trials and systematic reviews have suggested no benefit to preoperative drainage. Preoperative biliary stenting is generally indicated for evidence cholangitis, complications of jaundice, coagulopathy, or anticipated delay to surgery, such as with the use of neoadjuvant chemotherapy. Otherwise, routine drainage has not been shown to be useful.
Indications and Contraindications to Resection
More than 80% of patients with pancreatic cancer will present with unresectable disease, and the vast majority of patients undergoing successful resection experience local or distant recurrence. The primary factor determining management of newly diagnosed pancreatic cancers is therefore whether or not surgical resection can be obtained with a feasible chance at microscopically negative resection. In the past decade, several groups have attempted to define criteria of surgical resectability. Criteria of resectability vary slightly among these proposed classifications.
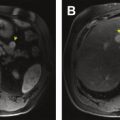
Resectable disease is generally classified as the lack of vessel involvement of major vessels (SMV, PV, common hepatic artery, superior mesenteric artery, celiac trunk) ( Box 1 ). Although the presence of an intact fat plane around vessels is often used to determine invasion, the lack of this radiographic finding is not universally consistent with invasion. Given the intimate association of the pancreatic parenchyma with the SMV and PV, tumors may frequently approach these vessels without invasion yet lack a radiographic fat plane. Descriptors such as “abutment” and “encasement” must often be interpreted cautiously as a result. In most classification systems, tumors are considered resectable with abutment of SMV or PV as long as there is no luminal narrowing or impingement ( Fig. 1 ). Interpretation of localized lymphadenopathy is important when determining resectability. For instance, with spread to the hepatic artery lymph node, patient survival is similar to stage IV pancreatic cancer.
- •
No locoregional vascular invasion
- ○
Intact portal vein/superior mesenteric vein a
- ○
No involvement of superior mesenteric artery
- ○
No involvement of common hepatic artery or celiac trunk
- ○
- •
No distant metastases
- •
No metastatic lymphadenopathy outside boundaries of planned resection
- •
Patient medically fit for major abdominal surgery
a Differs in various classification systems. Tumor deemed resectable if it abuts vein but vein is patent, if tumor abuts vein with no lumen narrowing or impingement, or alternatively if there is no vessel contact.
The term “borderline resectable” was popularized by Maurer and colleagues in 1999 and has evolved to include many tumors previously considered unresectable, such as those with more significant PV/SMV or superior mesenteric artery involvement. Resection of pancreatic tumors with venous reconstruction can provide survival benefits similar to resection without venous reconstruction, although these benefits have not translated into similar confirmed benefit with arterial reconstruction. As noted, classification schemes of borderline-resectable tumors differ somewhat, particularly pertaining to degrees of SMV and celiac artery involvement.
Unresectable tumors are currently defined as those with more extensive vascular involvement ( Box 2 ). All schemes consider encasement of the superior mesenteric or common hepatic artery to be unresectable disease ( Fig. 2 ). Extrapancreatic disease is widely accepted as a strict contraindication to attempted curative surgical resection.
- •
Locally unresectable tumor
- ○
Encasement of superior mesenteric or common hepatic artery
- ○
Invasion to inferior vena cava
- ○
- •
Distant metastases
- •
Metastatic lymphadenopathy beyond limits of resection
- •
Patient unfit for major abdominal surgery
Diagnostic Laparoscopy
Diagnostic laparoscopy has the potential to identify small peritoneal disease that would not be identified by standard imaging techniques, and laparoscopy has therefore been used in the past before planned surgical resection so as to avoid unnecessary laparotomy in the patient with occult metastatic disease. Occult metastatic disease has been described in up to 30% of patients on diagnostic laparoscopy, particularly in patients with locally invasive disease. Although the incidence of occult metastatic disease may decrease with improved imaging, CT and MRI have lower sensitivity for small-volume disease. An alternative strategy of selective diagnostic laparoscopy has been advocated given the falling incidence of occult metastases, with laparoscopy used particularly for patients with a higher risk of metastases, such as in distal pancreatic neoplasms or locally invasive tumors. Others have suggested a selective approach to diagnostic laparoscopy, with the use of laparoscopy for tumors larger than 3 cm; for all lesions in the neck, body, or tail of the pancreas ; or for patients with elevated preoperative levels of the tumor marker CA 19.9.
Technique/Procedure
Surgery for pancreatic cancer primarily consists of 2 distinct operations, largely based on the anatomic location of the tumor to the right or left of the SMV/PV axis: pancreaticoduodenectomy for tumors of the pancreatic head, and left-sided or distal pancreatectomy for tumors of the pancreatic body or tail. Detailed descriptions of these surgical procedures are well-described in the literature and are beyond the scope of this review. The basic steps of each procedure are outlined.
Pancreaticoduodenectomy: Technical Considerations
Surgical exposure for a pancreaticoduodenectomy (Whipple procedure) is first obtained either through a vertical midline incision or bilateral subcostal incision. Several fundamental steps must be accomplished.
Exposure of anterior and posterior pancreatic head
An advantage of mobilizing the pancreatic head and duodenum early in the procedure is the ability to assess posterior invasion and more importantly to allow easier vascular control of the PV and SMV by direct compression. The peritoneum along the lateral duodenal border is divided from the porta hepatis to the third portion of the duodenum when the SMV is encountered, to allow sharp dissection underneath the pancreatic head and over the inferior vena cava.
Exposure of superior mesenteric vein
Traditional descriptions of pancreaticoduodenectomy have emphasized the importance of determining resectability of the tumor early in the operation by assessing involvement of the SMV and PV with tumor. As SMV/PV involvement is not currently considered a strict contraindication to resection, other surgeons therefore delay any dissection underneath the pancreatic neck until later in the procedure, particularly when venous reconstruction is planned.
Dissection of porta hepatis
Special attention must be given to arterial variants, such as accessory or replaced right hepatic arteries at this juncture. The PV is easily exposed after division of the gastroduodenal artery and division of the bile duct. The antrum of the stomach (or the postpyloric duodenum) is divided.
Inframesocolic dissection
The proximal jejunum is divided, dissected from the mesentery, and transferred beneath the mesenteric vessels to the right side of the abdomen.
Dissection of pancreatic head from mesenteric vessels
The pancreatic neck is sharply divided over the SMV-PV, and the specimen is dissected from the SMV and superior mesenteric artery. The specimen is oriented, and sent to pathology with or without frozen section analysis of the pancreatic neck margin.
Reconstruction
Pancreaticojejunostomy is commonly performed with a duct-to-mucosa technique, although others prefer a technique of invagination of the pancreatic neck into the jejunum. A pancreatic duct stent is preferred by some to reduce pancreatic leak, although 2 meta-analyses have demonstrated that pancreatic duct stents do not change the rate of pancreatic fistula. Anastomosis of the pancreatic stump to the posterior wall of the stomach is commonly used in some centers, with some studies demonstrating decreased risk of pancreatic fistula with pancreaticogastrostomy. The hepaticojejunostomy is performed downstream from the pancreatic anastomosis in an end-to-side manner. Enteric continuity is established downstream from the hepaticojejunostomy, either within the lesser sac or in an antecolic manner.
Pancreaticoduodenectomy: Additional Considerations
Pylorus preservation
As classically described, pancreaticoduodenectomy entails removal of the pylorus and distal stomach. Pylorus-preserving pancreaticoduodenectomy (PPPD) was initially proposed as means of reducing the incidence of marginal ulceration at the gastroenterostomy. Some have argued that PPPD may exacerbate the common postoperative complication of delayed gastric emptying. However, retrospective studies have not confirmed this finding.
PPPD has been compared with routine or standard Whipple in many studies, with outcome metrics including nutritional status, oncologic adequacy, morbidity, and surgical margins, with most studies suggesting that PPPD does not impair oncologic margins or increase morbidity. Furthermore, several randomized studies comparing these procedures also have been reported, demonstrating no difference in operative duration, mortality, length of stay, delayed gastric emptying, or oncologic outcome.
Vascular reconstruction
Although involvement of the SMV or PV with pancreatic tumors has previously been considered a contraindication to surgical resection, such involvement may be considered a function of anatomic proximity rather than necessarily of aggressive tumor biology. Improved survival with PV resection versus palliative bypass has been demonstrated in a randomized study of patients with PV involvement with pancreatic adenocarcinoma. Studies also have demonstrated increased frequency of postoperative complications after pancreaticoduodenectomy with vascular reconstruction. Despite increased standardization in the diagnosis of borderline-resectable pancreatic malignancy, variation remains even between high-volume pancreatic centers in terms of the degree of vascular involvement that constitutes a contraindication to surgery. Small defects in the SMV or PV may be closed primarily, otherwise reconstruction may be performed with an end-to-end anastomosis or vein grafts with internal jugular or left renal vein. Venous reconstruction is increasingly performed at high-volume centers for borderline-resectable tumors, although arterial reconstruction is shown to be associated with poor short-term and long-term outcomes, and is justified in only highly selected patients.
Distal Pancreatectomy: Technical Considerations
Unlike tumors in the pancreatic head or neck, tumors in the body and tail of the pancreas do not require interruption and reconstruction of the bile duct or intestinal tract, nor is a pancreatic anastomosis required. The pancreas is removed left of the midline, up to or just to the right of the SMV/PV if necessary. Surgical mortality is considerably lower than with pancreaticoduodenectomy, although morbidity remains considerable with rates close to 50%; pancreatic leak or fistula from the pancreatic stump is one of the most commonly encountered complications.
Splenectomy is often performed concurrently with distal pancreatectomy due to the close relationship between the pancreatic parenchyma and splenic artery and vein. Pancreatic resection performed while sparing these vessels could potentially lead to compromised surgical margins; thus, a vessel-sparing approach may be better suited to surgery for premalignant or benign disease. Distal pancreatectomy with spleen preservation is described with a technique to maintain spleen perfusion via the short gastric vessels off the greater curvature of the stomach. Surgical splenectomy carries a small risk of postsplenectomy sepsis, and presplenectomy vaccination is recommended for pneumococcus, Haemophilus influenzae , and Neisseria meningitidis.
Open surgical exposure for distal pancreatectomy can be obtained either through a subcostal or midline incision. For either an open or minimally invasive approach, surgical steps are similar. The lesser sac is entered via the gastrocolic ligament between the stomach and the colon to expose the anterior pancreas. The splenic artery is isolated and divided on the anterior border of the pancreas, and the splenic vein is divided on the inferior surface of the pancreas. The pancreatic parenchyma may be divided either with surgical staplers, or sharply followed by mattress suture closure of the divided end. The remainder of the pancreatic parenchyma can then be dissected off the retroperitoneum with or without the spleen. Alternatively, the pancreatic tail and spleen can be mobilized from the retroperitoneum in a left-to-right manner before dividing the splenic vessels.
Fistula from the pancreatic duct is the most common complication after distal pancreatectomy. Although single-institution studies have suggested decreased rates of pancreatic fistula by using various techniques such as staple line mesh reinforcement, no method of pancreatic transection is universally demonstrated to be superior. Direct identification and suture control of the pancreatic duct may decrease the incidence of pancreatic leak, although identification of the duct may be challenging, particularly after stapled transection.
Strasberg and colleagues describe a modification of distal pancreatectomy referred to as “radical antegrade modular pancreaticosplenectomy” or RAMPS, with a goal of optimizing lymph node dissection and radial margins. The right-to-left dissection is primarily modified by ensuring a sufficiently deep posterior dissection behind anterior renal fascia and with or without removal of the adrenal gland and Gerota’s fascia. Although these methods have not been compared in a randomized manner to standard techniques, single-institution studies report favorable 5-year survival compared with historical controls.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree