To optimize the therapeutic value of an operation for cancer, surgeons must weigh survival value against mortality/morbidity risk. As a result of several prospective, randomized trials, many surgeons feel that international opinion has reached a consensus. Reflexively radical surgical hubris has certainly given way to a more nuanced, customized approach to this disease. But issues remain. This article critically reviews existing data and emphasizes areas of continued controversy.
To optimize the therapeutic value of an operation for cancer, surgeons must weigh survival value on one hand against mortality/morbidity risk on the other. As a result of several prospective, randomized trials, many surgeons view the muddied waters of international opinion concerning optimal gastric cancer treatment as having been filtered clean. But does this view withstand detailed scrutiny? Unquestionably, the reflexively-radical surgical hubris of yore has given way to a more nuanced, customized approach to this disease. Emphasizing existing trial findings and controversies, this review hopes to illuminate the topic so the reader can reach his own conclusions.
East-West differences in diagnosis and staging
East-West differences in gastric cancer have been commented on but it is not always realized that even the histologic diagnosis of gastric cancer varies. For example, noninvasive mucosal disease is routinely categorized as early gastric cancer according to the Japanese General Rules for Gastric Cancer Study, but as simple dysplasia or carcinoma in situ by Western pathologists. Western pathologists are reluctant to diagnose gastric cancer unless they identify microscopic invasion of the basement membrane lamina propria. The so-called Vienna Classification explicitly documents and addresses these major diagnostic differences. The new Group Classification incorporated into the Japanese Classification of Gastric Carcinoma (see later discussion) classifies lesions into 5 categories, from benign to obviously neoplastic, and this should remedy the problem.
Complications such as obstruction, bleeding, or perforation only infrequently prompt an emergency operation for gastric cancer. In the modern era, clinical staging drives treatment and such treatment is customized. Extent-of-disease evaluation for gastric cancer generally includes upper endoscopy with biopsy, endoscopic ultrasound, spiral computed tomography (CT) of the abdomen/pelvis, and a chest radiograph or chest CT. Laparoscopy has a role in the staging and treatment of selected cases, particularly in ruling out peritoneal dissemination or extraregional disease. Positron emission tomography (PET) scanning for gastric cancer (as opposed to esophageal cancer or gastroesophageal junction cancer) is controversial. The performance characteristics of fluorodeoxyglucose (FDG)-PET scanning for gastric cancer are not as good as for other neoplasms. For example, only 60% to 75% of primary gastric tumors have a suspicious standardized uptake value.
The current seventh edition of American Joint Commission for Cancer (AJCC)/Union for International Cancer Control (UICC) staging, to be used for all cases after January 1, 2010, represents a major departure from previous versions. For example, tumors arising from the proximal 5 cm of the stomach but extending to the gastroesophageal junction, as well as all esophagogastric junction tumors, are now classified for staging purposes as esophageal cancer (ie, not stomach). Combined with other changes, this has created substantial migration of both site and stage, compared with previous editions. Unintended consequences relating to treatment have appeared. These consequences will likely be addressed in future AJCC/UICC editions. For the purpose of this article, gastric adenocarcinoma is staged/classified according to modern seventh edition mandates.
A second staging issue should be highlighted. The Japanese Gastric Cancer Association (JGCA) staging (also termed the Updated General Rules, based on the title of the first English version published in 1981 ) represents an internationally popular alternative staging system used throughout Asia and many other areas of the world. This popular system, with its previously anatomic classification of lymph nodes, was initially designed not only to assess prognosis but to guide surgical treatment. This system has undergone extensive revision over the years as the recommended surgical treatment of regional lymph nodes has changed. It was not until 2001 that treatment guidelines began to be disconnected from the staging system. In 1997, with the publication of the 13th edition of the JGCA Staging Guidelines, the N4 (para-aortic) designation was eliminated, and, with it, the term D4 lymphadenectomy. With the change, the earlier N4 para-aortic disease became the newly defined N3 level. In the most recent 2011 version, the N3 category has also been eliminated. Given the redefinition of N2 nodes, a pre-1997 D3 lymphadenectomy is now a D2 lymphadenectomy. The modern D2 lymphadenectomy addresses regional node stations 1 to 12; nodal disease outside these node stations is now classified as metastatic M1 disease. To clarify the current definitions, the JGCA published an updated third English version in 2011, accompanied by separate algorithmic treatment guidelines, which should remedy the currently widespread international confusion. An additional advantage of this 2011 version is that final staging is now, for the first time, based on numbers of nodes involved and is in agreement with AJCC/UICC tumor- node, metastasis (TNM) staging definitions.
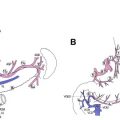
Modern JGCA D-level lymphadenectomy definitions are driven by whether a (mandated) total gastrectomy or distal gastrectomy is performed, based on primary tumor characteristics. For total gastrectomy, a D2 lymphadenectomy is now defined as complete removal of node stations 1 to 7, 8a, 9 to 11, and 12a. For DISTAL gastrectomy, D2 lymphadenectomy is now defined as complete removal of node stations 1, 3, 4sb and 4d, 5 to 7, 8a, 9, 11p, and 12a ( Fig. 1 ).

Extent of organ resection. Prospective randomized trails addressing routine total gastrectomy versus subtotal gastrectomy
The French Subtotal versus Total Gastrectomy Trial, by Gouzi and colleagues for the French Association for Surgical Research, was conducted between 1980 and 1985 to address the potential value of routine total gastrectomy versus the higher mortality and morbidity associated with this procedure, as documented by McNeer and others. Eligibility criteria included presence of an adenocarcinoma located in the distal half of the stomach, good organ function, and no evidence of nodal involvement higher than the gastroesophageal junction or in the splenopancreatic region. Cases of superficial carcinoma (in situ or early T1) were to be excluded, as were cases of obvious linitis plastica–type extensive infiltration within the gastric wall. Extensive lymph node dissection was not mandated, but proximal ligation/resection of the left gastric artery was. A Billroth II gastrojejunostomy reconstruction was used for all subtotal gastrectomy cases. Reconstruction for all total gastrectomy cases consisted of Roux-en-Y esophagojejunostomy. One-hundred and sixty-nine patients were randomized. In contrast with most other studies, this trial showed slightly lower mortality in the total gastrectomy group (1.3% vs 3.2%). Five-year survival rate for both groups was identical at 48%.
The Italian Subtotal versus Total Gastrectomy Trial was conducted from April 1982 to December 1993. Six-hundred and eighteen patients with localized gastric adenocarcinoma of the antrum were randomized to subtotal gastrectomy versus total gastrectomy. A D2 lymphadenectomy and Japanese-type omental bursectomy was recommended for all patients but not mandated. Inclusion criteria included histologic confirmation of adenocarcinoma, age less than 75 years, absence of serious comorbid conditions, and no history of previous malignancy, gastric surgery, or chemotherapy. During laparotomy, all patients were required to have a tumor-free proximal margin of 6 cm, and absence of any extraregional nodes, hepatic metastases, peritoneal metastases, or unresectable infiltration of contiguous organs. During this 1982 to 1993 period, 1372 patients from 31 Italian institutions were evaluated, and 648 randomized; after exclusions, 311 were left in the subtotal gastrectomy group and 296 in the total gastrectomy group. With a median 72 months’ follow-up, 5-year Kaplan-Meier survival was 65.3% for the subtotal gastrectomy group and 62.4% for the total gastrectomy group ( P = not significant [ns]).
The Hong Kong Trial of D1-Subtotal versus D3-Total Gastrectomy was conducted between October 1987 and December 1991 by Robertson and colleagues at the Prince of Wales Hospital in Hong Kong. The trial was open to patients undergoing laparotomy for grossly localized antral tumors that could be cleared to a 6-cm proximal margin with subtotal gastrectomy. Additional entry criteria included negative distal margin, absence of liver metastases, absence of peritoneal metastases, age less than 75 years, and absence of serious comorbid conditions. Neither intraoperative cytologic nor histologic analyses were performed. In the D3 group, distal pancreatectomy and splenectomy, and D3 lymph node dissection were routinely performed, but without omental bursectomy. The R-1-subtotal group underwent simple distal gastrectomy with a 6-cm proximal margin, high ligation of the right and left gastric arteries, and simple omentectomy, but no other node dissection. During the study period, 55 cases were randomized, 25 in the R-1 subtotal group and 30 in the D3 total group. In this trial, survival was better for the more simply treated R-1 subtotal group (median survival 1511 vs 922 days, P <.05). The D3 total group had longer operative time (260 vs 140 minutes, P <.05), more transfusions ( P <.05), and longer hospital stay (16 vs 8 days, P <.05). No patient in the D1 subtotal group died after surgery in hospital, in contrast with 1 patient in the D3 total group. ( P = ns).
Table 1 summarizes the results of these trials. Provided a negative-margin resection can be obtained with a subtotal gastrectomy, and provided it is not a linitis plastica situation (ie, diffuse infiltrating poorly differentiated cancer eliciting a leather-bottle stomach), routine total gastrectomy is to be avoided. The 2010 Japanese treatment guidelines, and the 2011 English version, call for a proximal margin of 3 cm from gross disease for localized well-circumscribed Borrmann I or II tumors, and a margin of at least 5 cm for other tumors. Total gastrectomy is performed whenever necessary to meet these requirements. Total gastrectomy should also be performed for cases of linitis plastica.
| Total vs Subtotal Trials | Inclusion Criteria | Mortality/Survival | Mortality/Survival | P Value (Survival) | |
|---|---|---|---|---|---|
| Subtotal | Total | ||||
| Gouzi et al | N = 169 | Antral tumor M-0 | 3%/48% (5-y survival) | 1%/48% (5-y survival) | ns |
| Bozzetti et al | N = 618 | >6 cm proximal margin possible M-0 | 1%/65% (5-y survival) | 2%/62% (5-y survival) | ns |
| Subtotal+D1 | Total+D = 3 | ||||
| Robertson et al | N = 55 | Antral >6 cm margin M-0, age <75 y | 0%/1511 d median survival | 3%/922 d median survival | 0.04 0.07 |
Extent of organ resection. Prospective randomized trails addressing routine total gastrectomy versus subtotal gastrectomy
The French Subtotal versus Total Gastrectomy Trial, by Gouzi and colleagues for the French Association for Surgical Research, was conducted between 1980 and 1985 to address the potential value of routine total gastrectomy versus the higher mortality and morbidity associated with this procedure, as documented by McNeer and others. Eligibility criteria included presence of an adenocarcinoma located in the distal half of the stomach, good organ function, and no evidence of nodal involvement higher than the gastroesophageal junction or in the splenopancreatic region. Cases of superficial carcinoma (in situ or early T1) were to be excluded, as were cases of obvious linitis plastica–type extensive infiltration within the gastric wall. Extensive lymph node dissection was not mandated, but proximal ligation/resection of the left gastric artery was. A Billroth II gastrojejunostomy reconstruction was used for all subtotal gastrectomy cases. Reconstruction for all total gastrectomy cases consisted of Roux-en-Y esophagojejunostomy. One-hundred and sixty-nine patients were randomized. In contrast with most other studies, this trial showed slightly lower mortality in the total gastrectomy group (1.3% vs 3.2%). Five-year survival rate for both groups was identical at 48%.
The Italian Subtotal versus Total Gastrectomy Trial was conducted from April 1982 to December 1993. Six-hundred and eighteen patients with localized gastric adenocarcinoma of the antrum were randomized to subtotal gastrectomy versus total gastrectomy. A D2 lymphadenectomy and Japanese-type omental bursectomy was recommended for all patients but not mandated. Inclusion criteria included histologic confirmation of adenocarcinoma, age less than 75 years, absence of serious comorbid conditions, and no history of previous malignancy, gastric surgery, or chemotherapy. During laparotomy, all patients were required to have a tumor-free proximal margin of 6 cm, and absence of any extraregional nodes, hepatic metastases, peritoneal metastases, or unresectable infiltration of contiguous organs. During this 1982 to 1993 period, 1372 patients from 31 Italian institutions were evaluated, and 648 randomized; after exclusions, 311 were left in the subtotal gastrectomy group and 296 in the total gastrectomy group. With a median 72 months’ follow-up, 5-year Kaplan-Meier survival was 65.3% for the subtotal gastrectomy group and 62.4% for the total gastrectomy group ( P = not significant [ns]).
The Hong Kong Trial of D1-Subtotal versus D3-Total Gastrectomy was conducted between October 1987 and December 1991 by Robertson and colleagues at the Prince of Wales Hospital in Hong Kong. The trial was open to patients undergoing laparotomy for grossly localized antral tumors that could be cleared to a 6-cm proximal margin with subtotal gastrectomy. Additional entry criteria included negative distal margin, absence of liver metastases, absence of peritoneal metastases, age less than 75 years, and absence of serious comorbid conditions. Neither intraoperative cytologic nor histologic analyses were performed. In the D3 group, distal pancreatectomy and splenectomy, and D3 lymph node dissection were routinely performed, but without omental bursectomy. The R-1-subtotal group underwent simple distal gastrectomy with a 6-cm proximal margin, high ligation of the right and left gastric arteries, and simple omentectomy, but no other node dissection. During the study period, 55 cases were randomized, 25 in the R-1 subtotal group and 30 in the D3 total group. In this trial, survival was better for the more simply treated R-1 subtotal group (median survival 1511 vs 922 days, P <.05). The D3 total group had longer operative time (260 vs 140 minutes, P <.05), more transfusions ( P <.05), and longer hospital stay (16 vs 8 days, P <.05). No patient in the D1 subtotal group died after surgery in hospital, in contrast with 1 patient in the D3 total group. ( P = ns).
Table 1 summarizes the results of these trials. Provided a negative-margin resection can be obtained with a subtotal gastrectomy, and provided it is not a linitis plastica situation (ie, diffuse infiltrating poorly differentiated cancer eliciting a leather-bottle stomach), routine total gastrectomy is to be avoided. The 2010 Japanese treatment guidelines, and the 2011 English version, call for a proximal margin of 3 cm from gross disease for localized well-circumscribed Borrmann I or II tumors, and a margin of at least 5 cm for other tumors. Total gastrectomy is performed whenever necessary to meet these requirements. Total gastrectomy should also be performed for cases of linitis plastica.
| Total vs Subtotal Trials | Inclusion Criteria | Mortality/Survival | Mortality/Survival | P Value (Survival) | |
|---|---|---|---|---|---|
| Subtotal | Total | ||||
| Gouzi et al | N = 169 | Antral tumor M-0 | 3%/48% (5-y survival) | 1%/48% (5-y survival) | ns |
| Bozzetti et al | N = 618 | >6 cm proximal margin possible M-0 | 1%/65% (5-y survival) | 2%/62% (5-y survival) | ns |
| Subtotal+D1 | Total+D = 3 | ||||
| Robertson et al | N = 55 | Antral >6 cm margin M-0, age <75 y | 0%/1511 d median survival | 3%/922 d median survival | 0.04 0.07 |
In situ and T-1 disease: role of endoscopic mucosal/submucosal resection
For a selected superficial early gastric cancer (ie, Tis or T-1 tumor), endoscopic mucosal resection (EMR) has emerged as a reasonable option. In the classic technique of EMR, a submucosal injection of saline floats the area of tumor-bearing mucosa off the underlying muscularis propria and the lesion is resected with a special cautery snare with hooks to preserve specimen orientation for margin analysis. The procedure can be technically challenging, but innovations such as use of incision endoforceps, aspiration mucosectomy, use of a stabilizing distal magnetic anchor, and use of double endoscope resection techniques can facilitate it. In endoscopic submucosal resection (ESR), the margins of resection are first circumscribed using a high-frequency electric knife and the submucosal layer specifically dissected from the muscularis propria layer.
Selection of cases suitable for EMR/ESR depends on the absence of disease in the regional lymphatics. A article paper by Gotoda and colleagues concerning 5265 surgically treated T-1 cases from the National Cancer Center Hospital and the Cancer Institute Hospital in Tokyo offers guidance. For intramucosal tumors, none of 1230 well-differentiated cancers of less than 30-mm diameter, regardless of ulceration findings, were associated with nodal involvement. Regardless of tumor size, for completely intramucosal tumors, none of 929 cancers without ulceration were associated with nodal metastases. For submucosal cancers, there was a significant correlation between tumor size larger than 30 mm and lymphatic-vascular involvement, with an increased risk of nodal involvement. However, none of the 145 well-differentiated adenocarcinomas of less than 30-mm diameter without lymphatic or venous permeation were associated with nodal involvement, provided that the lesion had invaded less than 500 μm into the submucosa.
In an 11-year, 445-case series by Ono and colleagues from the National Cancer Center Hospital in Tokyo, there were no gastric cancer–related deaths during a median follow-up period of 38 months (3–120 months). Although bleeding and perforation occurred in 5% of cases, there were no treatment-related deaths. When perforation occurs, if immediately recognized, the problem can be fixed with intraluminal application of endoclips, and the risk of intraperitoneal seeding associated with such an event seems negligible. For selected superficial T-1 cancers, EMR performed by experienced personnel can generate superb results and can be recommended, especially because any local recurrences can be addressed with salvage gastrectomy.
Laparoscopic resection with D1 lymphadenectomy and D1 pylorus-preserving gastrectomy represent valid options for T1 tumors not meeting EMR/ESR criteria. However, for all T1 tumors meeting EMR/ESR criteria, this is probably the appropriate choice, because the risk of intra-abdominal seeding can be avoided and salvage for positive margin or for recurrence is feasible. The current Japanese treatment guidelines echo these views.
Extent of lymphadenectomy: D1 versus D2
The Cape Town South Africa Trial (1982–1986) of D1 versus D2 lymphadenectomy was conducted between 1/’82 and 11/’86, by Dent and colleagues (this trial was termed Japanese R-1 vs Japanese R-2 at that time; the R term was subsequently replaced by the JGCA with D, to avoid confusion with the UICC R, a completeness-of-resection descriptor). Inclusion criteria included T1 to T3, N0 to N1 disease, no distant metastases, absence of significant comorbidity, and age less than 75 years. Patients from remote areas were excluded. For accurate staging, biopsies of celiac, common hepatic, and hepatic nodes and any abnormal nodes were taken for all patients. D2 (R-2 dissection in the nomenclature of the time) was performed according to the Japanese methods described by Kajitani ; specifically, removal of omentum, superior leaf of peritoneum on the transverse mesocolon, removal of the capsule of the pancreas (omentobursectomy, omental bursectomy, or bursectomy) and celiac-based lymph node dissection. For the gastric resection, gross proximal clearance of 5 cm was required in both arms, and reconstructive techniques were specified. During the period of study, 608 cases were reportedly evaluated, 403 were deemed surgical candidates, but only 43 (7% overall and 11% at laparotomy) were deemed to meet all eligibility criteria. Following treatment and discharge, patients were followed by examination at 3-month intervals. No attempt was made to screen for recurrence. No survival differences were noted. In-hospital mortality was zero for both groups. The trial did document increased operative time ( P <.005), increased blood transfusions ( P <.005) and longer hospital stay ( P <.05) for the D2 group. This single-institution trial was halted when single-institution accrual was deemed insufficient to adequately power the study.
The MRC Trial of Modified D1 versus Modified D2 Lymphadenectomy was conducted from 1986 to 1995, by Cushieri and colleagues of the Surgical Cooperative Group. In this trial, a British D1 procedure was defined in a manner that was different from the definition used by the Japan Research Society for Gastric Cancer (JRSGC). For this trial, a D1 lymph node dissection was one in which only lymph nodes within 3 cm of the tumor were removed (consistent with older TNM definitions of N-1 nodes). The D2 procedure was defined as one in which TNM N-2 nodes (ie, celiac, hepatoduodenal, retroduodenal, splenic, and retropancreatic nodes, depending on the location of the tumor, as well as perigastric nodes >3 cm from the tumor) were removed and the omental bursa resected (omentobursectomy). Distal pancreaticosplenectomy was performed almost exclusively in the D2 group, and splenectomy in both groups, but more frequently in the D2 group. Eligibility was assessed at staging laparotomy. Prelaparotomy exclusions included age less than 20 years and those with serious comorbid disease. All patients were assessed at laparotomy for the presence of peritoneal implants, liver metastases, and extraregional/peri-aortic adenopathy, particularly in the area of the left renal vein. Patients with disease in these sites were excluded. Intraoperative peritoneal cytology was not used. Eligible cases were deemed to have TNM stage I to III disease with negative margins of resection and a proximal margin of at least 2.5 cm free of gross disease. Of 737 cases registered, 337 were deemed ineligible at staging laparotomy because of advanced disease, leaving 400 cases for intraoperative randomization. With median follow-up of 6.5 years, 5-year overall survival for the D1 group was 35% versus 33% for the D2 group ( P = ns). Recurrence-free survival and disease-specific survival did not differ significantly. However, splenic resection, performed more frequently in the D2 group, and pancreatic resection, performed almost exclusively in the D2 group, seriously affected survival and proved to be independent predictors of poor survival. Complications and mortality were higher in the D2 group, and pancreaticosplenectomy was a powerful influence. The adverse impact of pancreaticosplenectomy, particularly pancreatectomy, confounded the lymphadenectomy question in this trial.
The Dutch Trial of D1 versus D2 Lymphadenectomy was conducted between August 1989 and July 1993 by surgeons participating in the Dutch Gastric Cancer Group. Eligibility criteria included age less than 85 years, adequate physical condition with no serious comorbid diseases, no previous cancer, no previous gastric surgery, and histologically confirmed gastric adenocarcinoma without evidence of distant metastases. Patients in both groups underwent distal or total gastrectomy according to the location of the tumor, with subtotal gastrectomy allowed if a proximal tumor-free margin of 5 cm could be achieved. At the onset of the trial, surgeons from 80 centers and 8 expert consulting surgeons were extensively instructed concerning Japanese-type surgical treatment according to JRSGC definitions and guidelines. Patients were randomized before surgery to arrange for the intraoperative presence of an expert consultant surgeon for all D2 cases. A Japanese expert surgeon attended every case during the first 4 months of the trial. The D1 procedure involved removal of all JRSGC-defined N1 nodes, generally the perigastric nodes at stations 1 to 6 along the greater and lesser curvatures of the stomach, along with removal of the lesser and greater omentum. The D2 procedure involved omentobursectomy (ie, removal of greater and lesser omentum, the superior leaf of the transverse mesocolon, and the capsule of the pancreas), frequent distal pancreatectomy and splenectomy (depending on tumor location), and removal of all JRSGC-defined N2 nodes at stations 7 to 12 (ie, left gastric, celiac, common hepatic, proper hepatic, and splenic artery, and splenic hilar nodes). Reconstruction following completion of the D2 node dissection was left to the local institutional surgeon, as was the postoperative care of the patient. Of the 1078 cases randomized before surgery, 82 (8%) were excluded for various reasons, most commonly unavailability of a consultant reference surgeon (35 cases), poor physical condition, or lack of histologic confirmation of the diagnosis. Of the remaining 996 patients randomized and entered into the study, 285 had evidence of incurable/extraregional disease at the time of surgery and were excluded. Seven-hundred and eleven deemed potentially curable underwent the randomly assigned treatment (ie, D1 or D2 resection) with curative intent. The 380 cases in the D1 group and the 331 cases in the D2 group were well balanced with respect to age, gender, tumor location, and tumor depth. Eighty-nine percent of the cases in each group were eventually shown to have undergone a pathologically confirmed, negative-margin, complete resection. A slightly higher proportion of cases in the D2 group underwent total gastrectomy (38% vs 30% in the D1 group). Among randomized cases, morbidity (25% vs 43%, P <.001) and in-hospital mortality (4% vs 10%, P = .004) were higher for the D2 group. With a median follow-up of 72 months, 5-year survival was 45% for the D1 group and 47% for the D2 group ( P = ns). Pancreatic and splenic resection, performed mostly in the D2 group (and mandated for particular tumor subsites) were associated with significantly higher morbidity and mortality in this study. Restricting the analysis to patients who did not undergo pancreatic or splenic resection (a post hoc, selected analysis), survival was higher for the D2 group (71% for the D2 group vs 59% for the D1 group, P = .02). Overall, given the operations defined, those who had a negative-margin resection deemed potentially curative had a risk of relapse at 5 years of 43% for the D1 group compared with 37% for the D2 group (difference between relapse rates was not significant). An 11-year follow-on report for this trial indicated that, of the 89 cases in the trial with pathologic N2 disease, there were 9 survivors after 10 years, and that 8 of the 9 were in the D2 group ( P = .01 for this post hoc analysis of the small N2 subgroup). Overall survival at the 11-year mark is 31% versus 35% for D1 and D2 respectively ( P = .53). A 2010 report with minimum 15-year follow-up on all patients clarifies that cumulative risk of death from gastric cancer is lower for the D2 group, but death from other causes is higher ( Fig. 2 ). In the final analysis, there is no overall benefit to routine pre-1997 D2 lymphadenectomy when pancreas-spleen resection is a mandatory part of the operation for some tumors ( Fig. 3 ).