Figure 14.1
Duodenal NET identified incidentally during endoscopy. Note the tumor originates in the submucosa and is seen as a small mass within the duodenal wall
14.1.2 Surgery
Patients who have ZES with localized, completely resectable tumors or those that cannot be identified by imaging are candidates for surgery. Patients who are referred with biopsy-proven duodenal NET are also candidates. Pernicious anemia and achlorhydria must be excluded, as they may represent an increased serum level of gastrin secondary to a lack of stomach acid (as discussed below regarding Type 1 gastric NETs). Similarly, medications that inhibit acid production (e.g., H2 receptor antagonists, proton pump inhibitors [PPIs]) will cause elevated levels of gastrin. Preoperative localization studies include CT or MR imaging of the pancreas and duodenum, as well as the liver, to exclude liver metastases. Somatostatin receptor scintigraphy (SRS; Octreoscan) is the single best imaging study. It images 90% of gastrinomas, and because it is a whole-body scan, it can detect distant metastases. It may miss small duodenal gastrinomas, however. A new type of nuclear medicine scan that appears to be more sensitive is 68Ga-DOTA-PET. Endoscopic ultrasound also may detect small duodenal tumors and lymph node metastases. Patients with ZES should be placed on PPIs to control acid hypersecretion. The usual dose of pantoprazole (Protonix) is 80 mg PO or IV twice daily. Patients with MEN1 and their families may have associated endocrinopathies, primary hyperparathyroidism, nephrolithiasis, prolactinoma, insulinoma, Cushing’s syndrome, NETs, and/or carcinoid syndrome. Clinical evaluation should exclude these conditions, especially primary hyperparathyroidism, which requires parathyroid surgery (usually removal of three and one half parathyroid glands) prior to the duodenal procedure.
Opening the abdomen and general exploration is commonly performed through a bilateral subcostal incision focused more on the right side of the abdomen than the left.
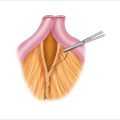
Duodenal NETs are often small (less than 1 cm). Consequently, these tumors are commonly missed on preoperative imaging and intraoperative dissection. Transillumination of the duodenum may be helpful. The tumor appears as an opaque mass within the wall (Fig. 14.2a). Opening the duodenum for transmural palpation is another method for operative detection. A Kocher maneuver is performed to mobilize the duodenum. We generally try to open the duodenum longitudinally and close transversely, so as not to narrow the lumen (Fig. 14.2b). If the tumor is diagnosed serendipitously by endoscopy before surgery, often the gastroenterologist will inject blue dye to mark the precise location of the tumor in the duodenum. Finally, during the initial exploration, the liver is also carefully evaluated for metastases by mobilization, palpation, and intraoperative ultrasound.
The next step involves mobilizing the entire right colon and hepatic flexure away from the duodenum, allowing better palpation of the duodenum. Duodenal NETs feel like small, firm nodules within the wall. They are usually mobile and feel like a pea. If the location of the tumor is not known, a duodenotomy (opening the duodenum) is essential because it is often the only way to visualize and palpate small duodenal NETs. This duodenal incision is used to explore the entire duodenum. The tumor originates in the submucosa and commonly dimples the mucosa. The surgeon can palpate the tumor within the wall of the duodenum with the index finger in the lumen and the thumb on the outside (Fig. 14.2b). Because the neoplasm arises from the submucosa and usually invades the mucosa, it cannot be effectively removed endoscopically and requires full-thickness excision with a rim of normal duodenum around the tumor.
In patients with MEN1, multiple duodenal NETs may be present, and the surgeon must carefully palpate and inspect the remainder of the inner surface of the duodenum to identify other neoplasms and plan a complete excision of all NETs. If the NETs are multiple and more extensive, or if it involves the ampulla of Vater and is larger than 2 cm, it may be more malignant and require a wider, more effective excision such as a pancreaticoduodenectomy (Whipple procedure). Do not confuse the ampulla of Vater or the entrance of the minor pancreatic duct with an NET. When uncertain, identification of the orifices of the pancreatic duct can be aided by the intravenous administration of secretin, which elicits pancreatic exocrine secretion. A cholangiocatheter placed through the cystic duct into the common bile duct and into the duodenum will also help to identify the ampulla.
After excising a duodenal gastrinoma, the duodenum is closed with a double-layer suture of full-thickness monofilament absorbable suture (e.g., PDS) and reinforced with a seromuscular layer of interrupted silk suture. In general, it is always closed in a transverse direction, so as not to narrow the lumen (Fig. 14.2b). If a long duodenotomy is necessary, a longitudinal closure may be performed, but it may narrow the duodenum, causing obstruction. For bulky tumors in the pancreatic head, tumors involving the pancreatic duct, or duodenal tumors involving the ampulla of Vater, a pylorus-preserving pancreaticoduodenectomy may be necessary.
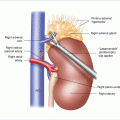
Following local excision of duodenal NETs, all lymph nodes within the region are excised in a systematic fashion starting with the hepatoduodenal ligament, the porta hepatis, and both the anterior and posterior border of the head of the pancreas (Fig. 14.3). The lymph node dissection is done after performing an extended Kocher maneuver in which the duodenum and the head of the pancreas are widely mobilized, as well as mobilization of the ascending colon, hepatic flexure, and transverse colon away from the head of the pancreas. This mobility of the duodenum and the pancreatic head allows dissection of all lymph nodes from both the anterior and posterior surface of the head of the pancreas. We have found that the harmonic scalpel facilitates bloodless lymph node excision. Quite commonly, the surgeon can find approximately five nodes embedded in the anterior head of the pancreas. The posterior nodes may be associated with enlarged lymph nodes between the inferior vena cava and the aorta, in the area of the left renal vein. These nodes should be excised as well. One can typically obtain five lymph nodes from the area posterior to the head of the pancreas and anterior to the vena cava. For the portal lymph node dissection, we identify and protect the common hepatic artery, the common bile duct, and the portal vein. We dissect lymph nodes around the origin of the common hepatic artery, along the right side of the common bile duct and underneath the duct close to the portal vein. Usually, about five nodes can be removed from the porta hepatis by dissecting along these structures. Complete pancreatic head, caval, and portal lymph node dissection is important because the tumors commonly spread to these lymph nodes. Detection of lymph node metastases suggests that the NET is malignant and portends a poorer prognosis.
The patient with ZES is kept on the same dose of PPI postoperatively for 3–6 months because parietal cell hypertrophy occurs, and acid hypersecretion decreases slowly. For ZES, a postoperative fasting serum gastrin measurement should be compared to the preoperative level. We typically wait 6 months before once again measuring all the tests—fasting serum gastrin, basal acid output, and secretin-stimulated gastrin—to diagnose ZES.
Postoperative complications include duodenal leak, bleeding, and pancreatitis. Duodenal stricture and tumor recurrence may be seen on long-term follow-up.
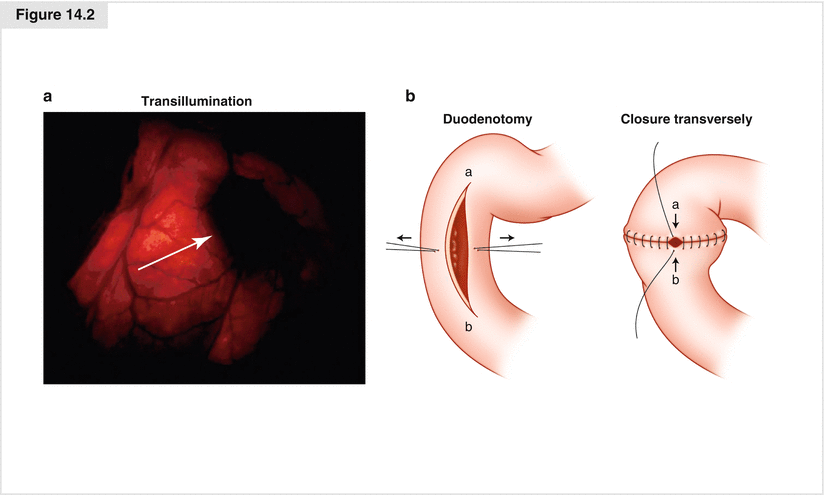

Figure 14.2




Duodenal neuroendocrine tumors: operative strategy. (a) Duodenal transillumination may show the tumor as an opaque mass (arrow). Duodenotomy allows better palpation. (b) Transillumination of the duodenum shows an NET as an opaque mass within the wall. After duodenotomy, the duodenum is usually closed transversely to avoid narrowing the lumen
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree