Fig. 4.1
Serous tubal intraepithelial carcinoma (STIC) at the fimbrial end of the Fallopian tube; the epithelial cells show enlarged and atypical nuclei with formation of tufts
While the concept of the fallopian tube epithelium being the origin of serous tumours is gaining popularity, it is suggested that most of the so-called ovarian primary non-serous epithelial tumours also have possible extraovarian origins. Endometrioid and clear cell tumours may actually originate from endometriotic tissue possibly from retrograde menstrual flow. Mucinous and Brenner tumours which are often linked to each other may originate from transitional-type epithelium found in paratubal and paraovarian locations [7, 9].
While the cell of origin of surface epithelial tumours remains a subject of debate, major advances have been made in the knowledge of the molecular basis of these tumours. These have highlighted that traditional morphology can predict specific molecular alterations likely to be present in a given tumour [10]. For example, p53 and BRCA mutations are commonly seen in ovarian high-grade serous carcinomas. Mucinous carcinomas are characterised by a high prevalence of KRAS mutations. Mutations of PIK3CA are observed frequently in clear cell carcinomas. Mutations of CTNNB1 are common in endometrioid carcinomas but rare in serous, mucinous and clear cell carcinomas.
It is thought that the candidate mechanism is oxidative genotoxic injury, perhaps a function of ovulation. Because ovulation occurs on the ovarian surface, both the ovarian surface and distal tube are targets [11, 12]. BRCA mutations and other genetic factors would increase the vulnerability to genotoxic injury and result in neoplastic transformation.
Given these advances in our understanding of the molecular alterations in the main types of ovarian epithelial malignancy, there has been a proposal for a dualistic pathway of ovarian epithelial carcinogenesis. This model divides the pathway of ovarian tumorigenesis into the type 1 pathway and the type 2 pathway [7]. Tumours in the type 1 pathway have in common frequent BRAF/KRAS mutations and rarely have a p53 mutation. Other mutations characteristic of this group are ERBB2, CTNNB1, PTEN, PIK3CA, ARID1A and PPP2R1A. They have lower cellular proliferation. These tumours are considered to arise via a well-defined adenoma-carcinoma sequence in a stepwise fashion and are genetically stable. These tumours have in common the fact that they all appear to arise from a benign precursor lesion. The cancers included within this group are low-grade serous, mucinous, endometrioid and clear cell carcinomas. All these tumours have a better 5-year survival but are not as chemosensitive as tumours in the type 2 group. Tumours in the type 2 pathway exhibit frequent p53 mutations and are genetically unstable. They show higher cellular proliferation, i.e. they are high-grade tumours which arise from less clearly defined precursor lesions. They have a poorer 5-year survival but are in general more chemosensitive compared to tumours in the type 1 group. They include high-grade serous carcinomas, carcinosarcomas and undifferentiated carcinomas.
Serous Tumours of the Ovary
General Features
These tumours are the commonest subtype of epithelial tumours. They are composed of cells which are ciliated or secretory and resemble fallopian tube epithelium (Fig. 4.2). These tumours are morphologically similar to tumours arising in the fallopian tube and müllerian epithelial rests in the pelvis (primary peritoneal carcinomas).
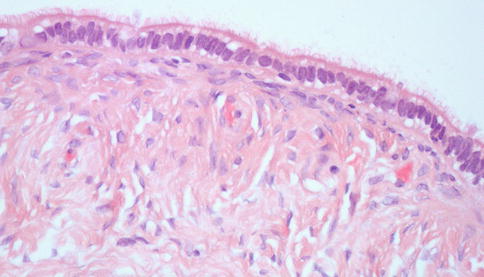

Fig. 4.2
Normal serous-type epithelium is composed of columnar cells with a ciliated luminal surface
Serous Carcinomas
These tumours account for 80–85 % of all ovarian carcinomas; 95 % of stage III to IV tumours are serous carcinomas. Fewer than 5 % of serous carcinomas present at stage I [13].
Macroscopically, these tumours range in size from being undetectable to greater than 20 cm. Two-thirds of the tumours are bilateral. Most are solid and friable with areas of necrosis and haemorrhage. Variable proportions of cystic change are seen. On microscopy the tumours have a glandular, papillary and solid architecture. The glands are slit-like and irregular, and the papillae show complex branching. Solid areas are extensive in high-grade tumours. There are varying amounts of psammoma bodies, ranging from few to numerous (psammocarcinoma).
Currently a two-tier grading system is most widely used [14, 15]. This separates the tumour into two distinct subtypes with different genetic features, pathology and behaviour. Although low-grade and high-grade serous carcinomas follow independent pathways and are currently believed to be totally different biological entities, infrequently high-grade serous carcinomas evolve from low-grade tumours. Up to 3 % of high-grade serous carcinomas in one series were associated with low-grade components [7]. Molecular studies of low-grade and high-grade components showed identical KRAS mutations in a few cases, suggesting a clonal relationship between low-grade and high-grade tumours in select cases. The above findings show that a small minority of high-grade serous carcinomas may evolve from low-grade tumours.
Low-Grade Serous Carcinoma
Low-grade serous carcinomas are most often seen in conjunction with a pre-existing borderline tumour, either serous adenofibroma, typical serous borderline tumour or micropapillary borderline tumour. These tumours consistently show mutations of BRAF, KRAS or ERBB2. Very few tumours (8 % of all tumours) show p53 mutations.
Microscopy
These tumours show a micropapillary architecture and lack necrosis (Fig. 4.3). They often contain many psammoma bodies (Figs. 4.4 and 4.5). Stromal invasion is seen in the form of glands or papillae surrounded by a non-epithelial lined space. Large complex papillae are seldom seen. The cells are bland with round to oval nuclei showing evenly distributed chromatin, sometimes containing a prominent nucleolus (Fig. 4.6). The cells should not show more than threefold variation in nuclear size. Multinucleated giant cells and bizarre cells are not a feature. Mitotic rate is low (less than 12/10 hpf) [15]. Rarely these tumours show an inverted macropapillary pattern which may be mistaken for a borderline serous adenofibroma but can be recognised to be an invasive tumour by the space surrounding medium-sized papillae. These tumours are often associated with pre-existing benign or borderline tumours.
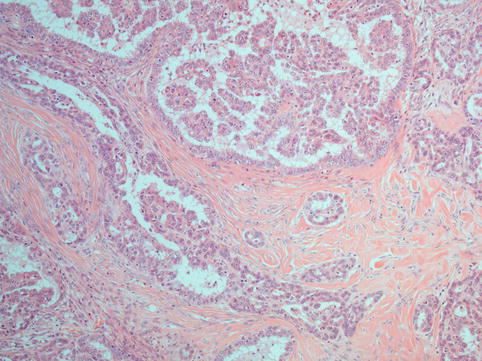
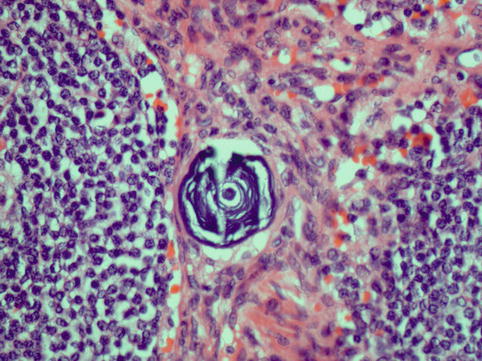
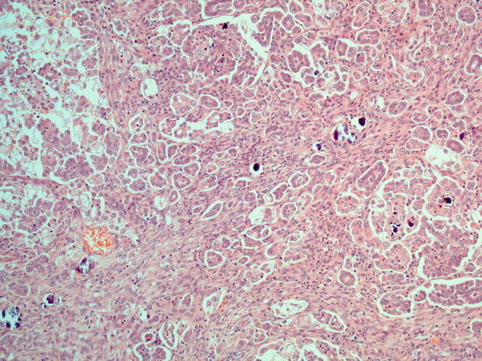
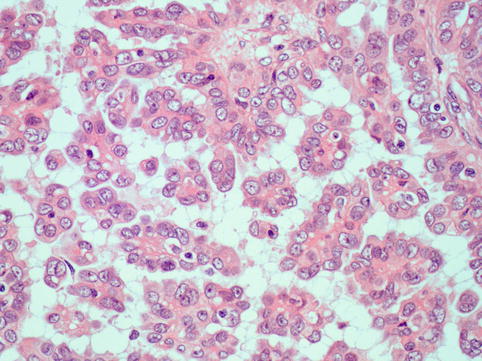

Fig. 4.3
Low-grade serous carcinoma is composed of tumour cell in a delicate papillary architecture

Fig. 4.4
Psammoma body; these are laminated calcified structures found in a variety of tumours which have papillary architecture, including serous ovarian carcinomas

Fig. 4.5
Low-grade serous carcinoma with numerous psammoma bodies

Fig. 4.6
Low-grade serous carcinoma: nuclei appear monotonous; though these can show some variation in size mitotic activity is low and atypia is not a feature
Immunohistochemistry of Low-Grade Carcinoma
These tumours are often WT1 positive and oestrogen (ER)/progesterone (PR) positive. The Ki-67 proliferation index is significantly lower in low-grade tumours compared to high-grade tumours (average, 23 %). p53 immunostaining (which is not the most reliable indicator of p53 mutations) is strongly positive in only 18 % cases. p16 is sometimes useful in differentiating between low-grade and high-grade serous carcinomas, and strong positivity (>75 % of tumour cells) is seen only in 27 % cases [16].
High-Grade Serous Carcinoma
The high-grade tumours are the more common type and constitute 90 % of all serous carcinomas [14]. These tumours are generally not seen adjacent to a borderline tumour. Roughly half these tumours are associated with intraepithelial carcinoma in the fallopian tube, almost always in the fimbrial end [17]. The rest of the high-grade serous tumours are believed to arise either from the ovarian surface or the peritoneum. These tumours are seldom associated with mutations of KRAS, BRAF or ERBB2. In contrast, p53 mutations occur in 50 % of these tumours. In addition, these tumours are associated with a high level of chromosomal instability. Another association is with germline BRCA1 or BRCA2 mutations. Roughly 15 % of ovarian serous carcinomas have a germline BRCA mutation, and almost all of these are high grade.
Microscopy
These neoplasms typically show complex papillae (Fig. 4.7). Psammoma bodies may be numerous, few or absent (Fig. 4.8). These tumours are often associated with variable amounts of necrosis. Other patterns encountered are glandular and solid and tumours with intracytoplasmic lumina and mucinous inclusions. They share high-grade nuclear features, large nuclei and often prominent nucleoli. Bizarre cells and multinucleated giant cells are often seen. Mitotic activity is high, and atypical mitotic figures are often seen (Fig. 4.9).
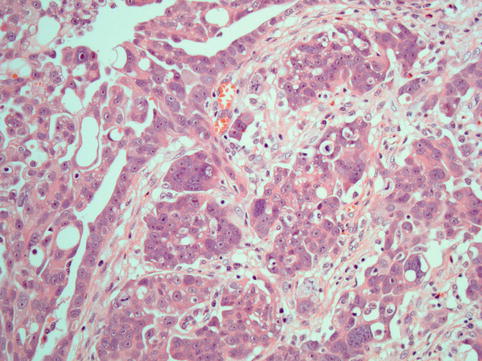
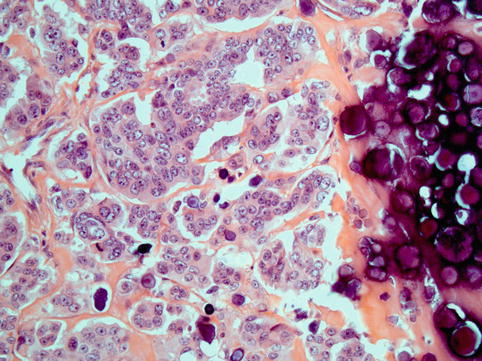
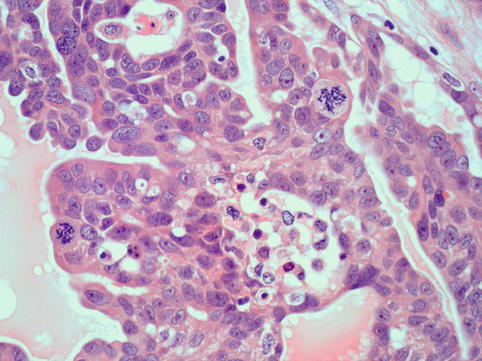

Fig. 4.7
High-grade serous carcinoma: In addition to papillae, the cells are arranged in solid masses

Fig. 4.8
High-grade serous carcinoma; large numbers of psammoma bodies may be seen

Fig. 4.9
High-grade serous carcinoma: cells have coarse chromatin and prominent large nucleoli and show frequent and atypical mitotic activity
Immunohistochemistry of high-grade carcinoma: These tumours are often WT1 positive and variably ER/PR positive. The Ki-67 proliferation index is significantly higher in high-grade tumours (average 55 %). p53 immunostaining is strongly positive in 64 % cases. p16 is strongly positive (>75 % tumour cells) in 83 % cases [16].
Serous Borderline Tumour
These tumours account for 10–15 % of serous tumours. They are tumours of low malignant potential with atypical proliferation of serous-type cells (accounting for more than 10 % of the total tumour) and no evidence of destructive stromal invasion. These tumours occur in patients 10–15 years younger than serous carcinomas (average age 45 years); however, the age range is broad (12–89 years). Borderline tumours of the ovary are associated with extraovarian disease in the form of peritoneal implants in 20–46 % of cases. The remaining cases are confined to the ovary. The prognosis of tumours confined to the ovary or those with associated noninvasive peritoneal implants is excellent (10-year survival between 92 and 100 %) [18]. The survival drops significantly if the implants are invasive [19].
Macroscopically serous borderline tumours (SBTs) are cystic with variable numbers of intracystic or surface excrescences. These excrescences are covered by fine papillae giving them a velvety appearance. They are more extensive than the smooth more polypoid excrescences of benign tumours. The tumours are bilateral in 25–40 % of cases. Microscopically the typical borderline tumours (which make up 90 % of all SBTs) are characterised by broad papillae showing hierarchical branching, covered by stratified epithelial cells showing mild to moderate atypia (Figs. 4.10 and 4.11). Mitotic figures are infrequent. The stroma is fibrous but may be oedematous. There may be surface involvement in up to 50 % of all cases. These surface tumours (auto implants) are identical to noninvasive extraovarian desmoplastic implants. Although autoimplants are seen more frequently in high-stage tumours (those with extraovarian disease), they do not alter the prognosis in stage I tumours.
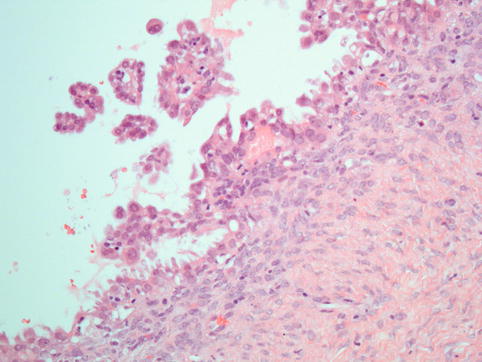
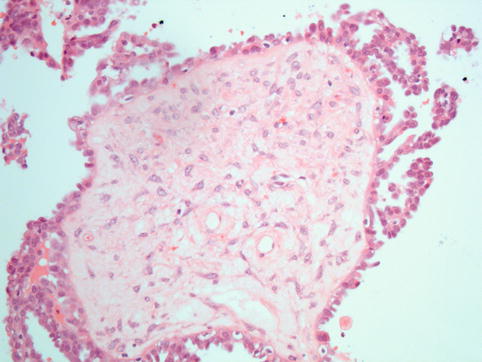

Fig. 4.10
Serous borderline tumour: the diagnosis depends on the presence of atypical proliferation in the form of tufts of cells and detachment of cell clusters into the luminal space, accompanied by cytological atypia

Fig. 4.11
Serous borderline tumour: tuft formation and cell detachment from the surface of a broad fibrous papilla
Stromal microinvasion (invasion <3 mm or <10 mm2 in greatest dimension) may be seen in 10–15 % of SBT as individual cells, small clusters of eosinophilic cells, cribriform nests or small papillary structures within the cyst wall or the stroma of papillae with minimal stromal response. This is in contrast to the destructive stromal invasion in serous carcinomas in which the invasion by neoplastic epithelium is irregular, haphazard and associated with effacement of ovarian stroma and/or a stromal fibroblastic reaction [20]. Microinvasion is more commonly seen in pregnancy (eight- to nine-fold more frequently). Until quite recently it was believed that microinvasion in a borderline serous tumour does not confer a worse prognosis; however, large studies have now shown that with the exception of microinvasion in SBT occurring in pregnancy, tumours with microinvasion have poorer clinical outcomes worse than those without [19, 21].
Approximately 10 % of serous borderline tumours show micropapillary morphology. These tumours are characterised by filiform, nonbranching micropapillae without fibrovascular cores which emanate from a fibrous stalk in a nonhierarchical ‘Medusa head-like’ pattern (Fig. 4.12). This proliferation can sometimes have a cribriform pattern. The tumour cells are monomorphic, cuboidal to columnar and sometimes show a hobnail appearance. They show mild to moderate atypia and often contain small nucleoli. The micropapillary area should be at least 5 mm in size before the tumour is labelled as a micropapillary variant. Micropapillary variants of SBT are different from typical SBT in that they are more often bilateral, have a higher incidence of extraovarian implants which may be invasive, have a higher frequency of surface involvement and a higher stage at presentation. These tumours have an overall poorer survival largely due to association with invasive implants. Although it has been suggested that micropapillary tumours be classed as cancers, currently the preferred term for these tumours is SBT with micropapillary features [22].
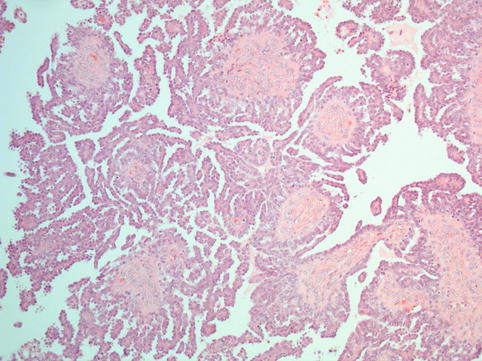

Fig. 4.12
Micropapillary pattern in a serous borderline tumour: the entire circumference of the fibrous papillae is covered by long and slender tufts of cells; this is also described as ‘nonhierarchical’ branching as the tiny micropapillae emanate directly from a broad fibrous papilla rather than the formation of progressively smaller calibre branches
Extraovarian implants are seen in 20–46 % of all SBTs [23]. They are more frequent in micropapillary variants and in borderline tumours with surface involvement. In fact, 94 % of patients with implants have borderline tumours with an exophytic surface component. The type of implant, invasive or noninvasive, is the most important prognostic factor in SBT. Over 80 % of implants are noninvasive; 10–15 % are invasive and 5 % are indeterminate.
Invasive implants are characterised by the presence of irregular, haphazard infiltration into normal tissue by glands or small solid epithelial nests (Fig. 4.13). Invasive implants sometimes engulf or replace omental adipose tissue. In addition to invasion of underlying normal tissue, a micropapillary architecture and solid epithelial nests surrounded by clefts are also considered by some as criteria for invasive implants [24].
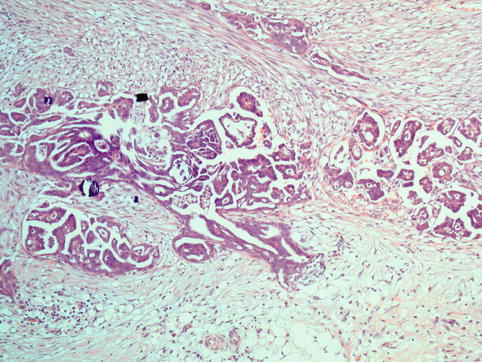

Fig. 4.13
Invasive implant from a case of serous borderline tumour: there is haphazard infiltration of adipose tissue by tumour-forming glands and papillae
Noninvasive implants are further classified as desmoplastic or epithelial, based upon the presence or absence of a marked stromal reaction. The noninvasive epithelial implants comprise glands and papillae which sometimes show hierarchical branching and epithelial tufting. They resemble the papillary proliferation of the primary borderline ovarian tumour with minimal or absent stromal response. Desmoplastic noninvasive implants are composed of peritoneal plaques and nodules composed of glands and papillae embedded in a fibroblastic stroma. The individual papillae and glandular structures are often associated with psammoma bodies. When the distinction between invasive and noninvasive implants is problematic, the implants are classified as indeterminate [19].
Although it has been established that it is the invasive implants that confer an adverse prognosis to serous borderline tumours, noninvasive implants also do impart a slightly greater risk of adverse outcome than when absent [19]. Noninvasive implants should be differentiated from endosalpingiosis, which is characterised by the presence of well-formed glands lined by tubal-type epithelium. Various theories regarding the origins of implants have been proposed. Transcoelomic spread from SBT is the most popular because of the frequent association with ovarian surface involvement and clonality studies showing similarities between ovarian tumours and implants [25]. Alternative theories that they develop from endosalpingiosis or synchronously directly from the peritoneum have also been suggested.
Lymph node involvement is seen in 20–30 % of patients with SBT who undergo a lymphadenectomy for staging. The lymph node involvement is typically in the sinuses and not in the lymph node parenchyma, a feature which helps to differentiate these from endosalpingiosis which is a close differential diagnosis. The presence of lymph node involvement does not alter the prognosis of these tumours [26].
Mucinous Tumours of the Ovary
This group of tumours (including their benign, borderline and malignant counterparts) represents the second most common type of epithelial tumours. These are characterised by mucin-containing epithelium that resembles either gastrointestinal epithelium (intestinal type) or endocervix (endocervical type) (Fig. 4.14). About 80 % of the tumours are benign, 10 % are borderline and 10 % are malignant. Occasionally, they are mixed with other types of surface epithelial neoplasms, including benign Brenner tumour. Three to five percent develop in association with dermoid cysts [27] . The most common molecular genetic alteration in mucinous borderline tumours and mucinous carcinomas is a point mutation of KRAS. In fact an increasing frequency of KRAS mutations at codons 12 and 13 is described in mucinous cystadenomas, mucinous borderline tumours and mucinous carcinomas, respectively, suggesting a stepwise transition from benign precursors [17].
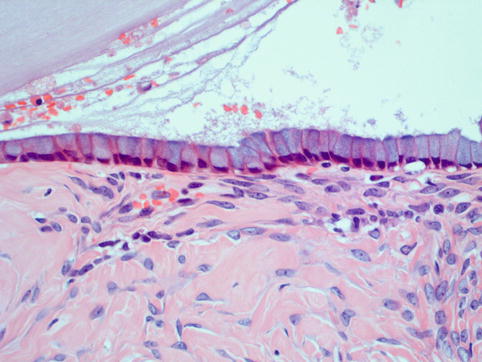

Fig. 4.14
Mucinous epithelium of intestinal type consists of mucin-secreting columnar epithelial cells with basally located bland nuclei
Mucinous Carcinoma
Primary ovarian mucinous carcinomas are relatively rare. The incidence is much lower than was previously believed to be the case. Mucinous carcinomas account for less than 3 % of all ovarian carcinomas [13, 28]. The main reason for the apparent reduction in incidence is the recognition of the fact that many of the tumours believed to be primary ovarian mucinous carcinoma in the past were in fact secondary adenocarcinomas. Also the distinction between a borderline tumour with intraepithelial carcinoma and microinvasion and a frankly invasive adenocarcinoma has become more clearly defined allowing many more tumours to be classified as borderline tumours [13]. Most tumours are stage I at presentation.
Macroscopically these tumours are variably cystic with solid areas. Microscopically the epithelium may be of gastrointestinal type or endocervical type. Most primary ovarian mucinous carcinomas are of gastrointestinal type [29]. These tumours are characterised by glandular and cystic spaces lined by columnar or polygonal epithelial cells with cytoplasmic mucin, sometimes containing goblet cells (Fig. 4.15). The endocervical-type tumours show papillae and glands lined by endocervical-type mucinous epithelium. Minor components of serous epithelium may be present, but, when these are prominent, the tumours are labelled seromucinous carcinomas. The endocervical-type mucinous tumours are often bilateral and may be associated with endometriosis. Mucinous tumours are graded using a three-tier grading system. Some use the Shimizu and Silverberg grading system [30]; others use a grading system similar to International Federation of Gynecology and Obstetrics (FIGO) grading of endometrioid carcinoma [31]. Most tumours (90–95 %) are morphologically heterogeneous with benign and borderline components present, and only rarely are they uniformly malignant. Mucinous tumours are considered invasive if one of two patterns of stromal invasion is present, infiltrative or expansile. An infiltrative pattern is defined as disorderly penetration of the stroma by neoplastic cells with or without an associated desmoplastic response. Infiltrative invasion >3 mm in two linear dimensions or with a total area >10 mm2 is considered frankly invasive. Tumours with less infiltration are classified as borderline with microinvasion. An expansile pattern is defined as a complex arrangement of glands, cysts or papillae lined by malignant epithelium with minimal or no recognisable intervening stroma.
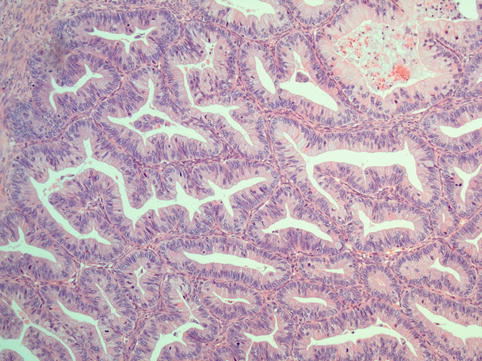

Fig. 4.15
Mucinous carcinoma of the ovary consists of glands lined by mucinous epithelium crowded together with no intervening stroma
A major consideration in the diagnosis of mucinous carcinomas and borderline tumours is the exclusion of metastasis from the gastrointestinal tract, including biliary tract and pancreas. Findings favouring a primary tumour over a metastatic carcinoma are unilaterality, size >10 cm, a smooth tumour surface with absence of surface or hilar involvement, benign-appearing or borderline-appearing areas (either with atypia only or with intraepithelial carcinoma), absence of extensive vascular invasion, absence of necrosis in the absence of torsion and finally an ‘expansile’ rather than a destructive infiltrative invasive pattern. Extensive intra-abdominal disease as well as a multinodular appearance of the tumour with intervening normal parenchyma favours a metastatic carcinoma, as does resemblance of the tumour to a known primary [32]. The possibility of metastasis should always be strongly considered in cases with a known extraovarian primary. The index of suspicion for an extraovarian primary should be high in a widely disseminated mucinous malignancy. It must be noted that algorithms based on size and laterality alone will identify about 85 % of cases correctly, but the remainder could be wrongly categorised so other features should be taken into account. Certain patterns, such as signet ring morphology and abundant extracellular pools of mucin, are almost exclusively seen in metastases (Figs. 4.16 and 4.17).
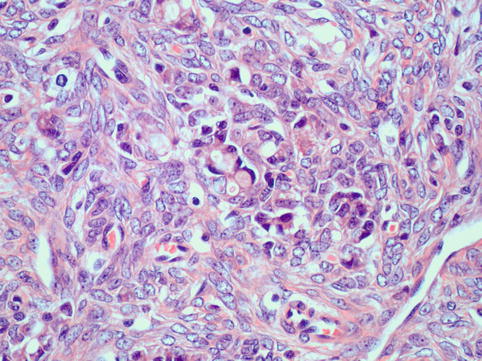
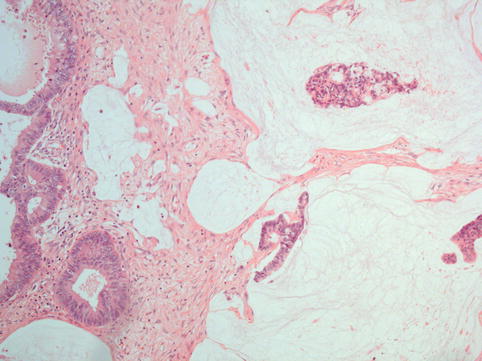

Fig. 4.16
Krukenberg tumour: Signet ring cell carcinoma shows very monotonous epithelial cells with eccentric nuclei displaced to the periphery by a large mucin vacuole (an example is seen at the centre of this field). Tumour cells are surrounded by a prominent reactive stromal component which may mask the true nature of this neoplasm

Fig. 4.17
Metastatic mucinous carcinoma within the ovary; a pattern characterised by abundant extracellular pools of mucin suggests extraovarian origin
Immunohistochemistry can help distinguish between primary and metastatic carcinomas from the gastrointestinal tract, although an overlap exists between the various markers. As stated, most primary ovarian mucinous carcinomas are of the intestinal or enteric type, and therefore they are negative for oestrogen and progesterone receptors and commonly positive for cytokeratin (CK) 20, carcinoembryonic antigen (CEA), cancer antigen (CA) 19.9 and CDX2. CA 19.9 is often diffusely positive while CK20, CEA and CDX2 are most commonly focally positive, but exceptions may sometimes exist where these markers are either negative or even diffusely positive. Positivity with these markers may be reflected in increased serum levels. For example, serum CA 19.9 may be massively elevated in primary ovarian mucinous neoplasms of intestinal type. These tumours are usually, although not always, diffusely CK7 positive. Although most colorectal carcinomas are CK7 negative, rectal carcinomas may often be CK7 positive [29]. Racemase and beta-catenin (β-catenin) may also be useful markers as they are more often positive in metastatic colorectal carcinomas compared to primary ovarian carcinomas [33]. SMAD4 can sometimes be useful in differentiating ovarian mucinous tumours from metastatic pancreatobiliary tumours as it is almost invariably positive in primary ovarian mucinous neoplasms while approximately 50 % of pancreatic and some biliary tract adenocarcinomas are negative. p16 is positive in endocervical tumours and negative in primary mucinous carcinomas. Oestrogen receptor (ER) is positive in metastatic breast carcinomas and endometrioid tumours.
Mucinous Borderline Tumour
Macroscopically mucinous borderline tumours (MBTs) are large multiloculated cysts (mean diameter, 17 cm) with fleshy polypoid areas, excrescences or sometimes solid areas. Around 90 % of MBTs are unilateral [23, 27]. Microscopically, as with invasive mucinous carcinomas, MBT can be either of intestinal or endocervical type.
The endocervical-like-type tumours are closely related to serous borderline tumours with which they are often mixed (also known as seromucinous borderline tumours or müllerian borderline mucinous tumours). These account for only 5–14 % of müllerian borderline mucinous tumours and have little in common with intestinal-type mucinous tumours. These tumours are often associated with background endometriosis and may even arise from an endometriotic cyst. They are characterised as broad papillae covered by endocervical-type epithelium admixed with rounded or polygonal cells with abundant eosinophilic cytoplasm (so-called indifferent cells) which are commonly seen at the tips of the papillae. Also seen are numerous acute inflammatory cells within the cytoplasm and in the extracellular mucin. The neoplastic cells show minimal nuclear atypia, but rare cases of intraepithelial carcinoma and microinvasion are reported which have overall benign behaviour. Around 6 % of these tumours are associated with noninvasive or invasive implants in the peritoneum and rare lymph node metastasis [34, 35]. These tumours are bilateral in 15–40 % of cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree