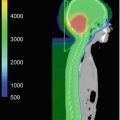
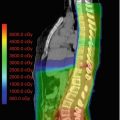
Fig. 7.1
Representative axial images from MRI of a 4-year-old patient with SPNET. On non-contrast T1-weighted (a), T2-weighted (b), and fluid-attenuated inversion recovery (FLAIR, c) images, tumor has isointense signal equivalent to normal gray matter, whereas the tumor demonstrates abnormal diffusion restriction on diffusion-weighted imaging (DWI, d) due to high cellularity of the tumor. Note characteristic absence of associated vasogenic edema on T2-weighted and FLAIR images
7.5 Workup
Due to a high predilection for central nervous system PNET to disseminate in the cerebrospinal fluid (CSF), with incidence of CNS metastasis identified in up to one-fourth to one-third of patients at diagnosis (Jakacki 2005), workup of patients suspected of having SPNET includes complete imaging of the neuraxis, preferably with gadolinium-enhanced MRI, and assessment of CSF cytology (Terterov et al. 2010). While there is some debate as to the optimal timing and method of CSF sampling, due to risk of both false positive and false negative results (Terterov et al. 2010), pathologic analysis of CSF should be evaluated prior to surgery or at least 10 days following resection of the primary tumor for staging. Postoperative MRI of the brain within 48 hours of surgery is also necessary to assess extent of tumor resection, and thereby determine the risk grouping of the patient (Chintagumpala et al. 2009). The risk of extracranial spread of PNET is rare, but complete metastatic evaluation includes bone scintigraphy, bone marrow biopsy, and imaging of lungs, liver and nodal regions to confirm the absence of distant spread of disease to these sites.
7.6 Acute Management
Given the preponderance of SPNET to present as large tumors with associated symptoms of increased intracranial pressure, acute management of patients often includes external ventricular drain placement for hydrocephalus and medical management of seizure. The benefit of steroids in SPNET is less clear, since these tumors tend not to have associated vasogenic edema despite significant mass effect (see Fig. 7.1).
7.7 Treatment
Because of the relative rarity of SPNET, the current standard therapy for patients with this diagnosis has been extrapolated from that for the more common medulloblastoma and combines maximal safe resection and adjuvant radiotherapy and chemotherapy (either concurrent or sequential), ideally to be initiated within 30 days of surgery.
7.7.1 Evolution to Current Practice
7.7.1.1 Role of Surgery
The principle role of surgery in the management of SPNET is for pathologic diagnosis and cytoreduction, but because of the high propensity for SPNET to disseminate throughout the neuraxis, this disease is considered not surgically curable (Jakacki 2005). Results of both prospective and retrospective cohorts of SPNET subjects have revealed both positive and negative correlation between extent of tumor resection and outcome.
In a report of the subgroup of 44 pathologically confirmed SPNET (including pineoblastoma) subjects enrolled on the prospective Children’s Cancer Group (CCG) study of high-risk PNET, CCG-921, only a minority had gross total resection (GTR) of tumor (37% non-pineal and 18% pineal SPNET) (Cohen et al. 1995). Excluding patients with known metastatic disease or pineal primary tumor, extent of resection was not found to be associated with progression-free survival (PFS) in the SPNET cohort, although impact of surgery on neurologic function and quality of life (QOL) was not assessed (Cohen et al. 1995). In the combined HIT 88/89 and HIT 91 trials in Germany and Austria, the survival outcomes of 63 SPNET (52 of which were non-pineal) patients treated with multimodality therapy were comparable for the 1/3 who had GTR compared with the remaining 67% who had subtotal resection (STR) (Timmermann et al. 2002). Analysis of 68 eligible patients with SPNET (54 of which were non-pineal) enrolled on the PNET 3 study conducted by the Société Internationale d’Oncologie Pédiatrique (SIOP)/United Kingdom Children’s Cancer Study Group (UKSCCG) also failed to demonstrate statistically significant improvement in event-free survival (EFS) or overall survival (OS) following GTR (Pizer et al. 2006). Similarly, in a retrospective series of 48 SPNET pediatric patients from Canada, at least 90% resection (with residual <1.5 cm2) was not found to have improved survival compared with lesser extent of resection (Johnston et al. 2008).
An early retrospective analysis evaluated 36 patients with SPNET (26 of which were non-pineal), who were treated at Hospital for Sick Children in Toronto (Dirks et al. 1996). In this series, there was a trend for improved outcomes in patients undergoing GTR, and half of those still alive at last evaluation had complete resection. The median survival was very poor at only 23 months for this entire group, which may be attributed, at least in part, to 50% of patients being less than 3 years of age and only a minority receiving chemotherapy. In a series of 22 consecutive patients with SPNET (nine of which were non-pineal), 5-year PFS rate was doubled from 25% in patients undergoing biopsy/STR to >50% in those undergoing near or gross total resection. The benefit of more aggressive surgery did not reach statistical significance in this analysis due to small patient numbers (Reddy et al. 2000).
Except where noted, the analyses above primarily included subjects at least age 3 years or greater. In the combined Pediatric Oncology Group (POG) 9233/34 randomized controlled study of dose-intensive (DI) versus standard chemotherapy for patients younger than 3 years of age, GTR was associated with significantly improved EFS of 40% in the subset of 29 patients with non-metastatic SPNET (Strother et al. 2014).
While the studies above included mixed populations of pineal and non-pineal SPNET, a comprehensive literature review of over 100 studies of 299 patients with pineoblastoma demonstrated that 5-year survival rates improved from 29% following debulking to 53% with STR, and to 84% with GTR (log-rank P < 0.001, (Tate et al. 2012)). The importance of GTR was confirmed on multivariate analysis, where effects of patient age, M stage, and other adjuvant therapies were taken into account. Of note, this analysis did include young adult patients (mean age 28 years) who comprised the cohort treated with surgery followed by radiation therapy (RT). In a retrospective series of 11 patients with pineoblastoma from Children’s Hospital of Boston/Dana-Farber Cancer Institute, three of these patients who had GTR were disease free at 1-year post diagnosis, whereas half of the remaining eight patients were alive >6 months from diagnosis. This finding suggested a trend for improved outcomes relative to greater extent of resection (Gilheeney et al. 2008).
The preponderance of data supports aggressive surgery to improve outcomes for both non-pineal and pineal SPNET.
7.7.1.2 Role of Chemotherapy
Standard Chemotherapy
Given a high predilection for dissemination, chemotherapy has been utilized as part of aggressive multimodality therapy for SPNET. While these tumors have been found to be sensitive to chemotherapy, particularly in infants and very young patients in whom deferral of RT is considered preferential in order to avert or delay potential adverse late sequelae of radiation, the response of SPNET to chemotherapy has been found to be of short duration (Jakacki 2005).
Although early studies examined single agent efficacy in medulloblastoma, combination drug regimens and timing of chemotherapy have been evaluated in subjects with the clinically more aggressive SPNET in multiple prospective clinical trials. The CCG-921 study randomized half of the 44 confirmed SPNET subjects to standard eight cycles of chemotherapy with CCNU, vincristine (VCR), and prednisone after surgery and craniospinal irradiation (CSI) versus two postoperative cycles of 8-in-1 chemotherapy with methylprednisolone, CCNU or carmustine, VCR, procarbazine, hydroxyurea, cisplatin (CDDP), cytarabine, and cyclophosphamide (CYCLO), followed by CSI and an additional eight cycles of 8-in-1 therapy (Cohen et al. 1995). Three-year PFS and OS were 45% and 57%, respectively, and were not statistically different between subjects treated with the two chemotherapy regimens, although the 8-in-1 had greater toxicity (predominantly hematologic). The subset of patients with pineal tumors had significantly improved 3-year PFS and OS (61% and 73%, respectively) compared with non-pineal SPNET.
Timing of chemotherapy was also assessed by a single-arm pilot trial and phase 2 trial of intense, multi-agent chemotherapy prior to RT (CSI plus boost) conducted by the German HIT group (HIT 88/89) that included ten subjects with SPNET (Kuhl et al. 1998). The chemotherapy regimen included up to two cycles of procarbazine, ifosfamide (IFOS) with mesna, etoposide (ETOP), high-dose methotrexate (MTX), CDDP, and cytarabine (Ara-C). Five-year overall response rate (complete response [CR] plus partial response [PR]) in the SPNET cohort was 57%, although the 5-year PFS of 20% was lowest in this subset of patients and was worse than observed in the CCG-921 study. The follow-up expansion study HIT 91 randomized a total of 515 pediatric patients aged 3–18 years with PNET to the pre-radiation chemotherapy regimen of HIT 88/89 or to standard therapy with concurrent VCR and CSI plus boost followed by eight cycles of CCNU, CDDP, and VCR. Of the 515 patients, 53 were found to have SPNET, and 30 of these received the experimental HIT 88/89 therapy (Timmermann et al. 2002). In the combined analysis of all 63 SPNET subjects on the HIT 88/89 and HIT 91, almost 2/3 had disease progression, and nine of the 10 patients who progressed during adjuvant therapy were treated with pre-radiation chemotherapy. The 3-year PFS for the combined groups was 39%, and median time to progression was 10 months (Timmermann et al. 2002). Thus, while the early HIT 88/89 studies demonstrated sensitivity of SPNET to aggressive multidrug chemotherapy, outcomes in these patients were not improved using this approach. Of note, only half of the children with SPNET in HIT 91 were randomized due to parental refusal.
The SIOP/UKCCSG PNET 3 study was designed to evaluate the efficacy of four alternating cycles of VCR/ETOP/carboplatin (CBDCA) with VCR/ETOP/CYCLO combination chemotherapy prior to RT versus adjuvant RT alone in pediatric subjects ≥3 years of age with SPNET (Pizer et al. 2006). Addition of chemotherapy prior to RT was associated with increased toxicity, especially grade 3–4 drop in blood counts, but had no impact on EFS or OS compared to adjuvant RT alone. It is worth noting, however, that, of the 68 eligible SPNET patients on this study, only 13 were randomized according to the protocol specifications.
Chemotherapy for Very Young Patients
Although addition of chemotherapy prior to RT did not show clear benefit in patients 3 years or older, several prospective studies investigated aggressive multi-agent chemotherapy regimens in the very young as a means to delay or omit RT in this vulnerable population. In the highly cited Pediatric Oncology Group (POG) study of adjuvant chemotherapy for children with malignant brain tumors under 3 years of age (“Baby POG”), Duffner et al. reported on the outcomes of 198 children treated with two cycles of CYCLO and VCR followed by one cycle of CDDP and ETOP for 1–2 years, at which time RT was permitted (Duffner et al. 1993). Thirty-six (18%) of these children had SPNET, and of these, 22 were <2 years old at diagnosis. Only eight of 30 of these children who underwent surgery had GTR of tumor, the only factor identified on multivariate analysis to be associated with improved PFS. Among all the histologies included in this analysis, the children with SPNET had the worst outcomes, with 2-year PFS and OS rates of 19% and 21%, respectively. Most tumor recurrence in these children occurred within the first 12 months of treatment. Given this finding, use of this chemotherapy regimen was not recommended for young children with SPNET (Duffner et al. 1993).
The Société Française d’Oncologie Pédiatrique (SFOP) also conducted a study of postoperative multi-agent chemotherapy alone for children <5 years of age with malignant brain tumors. In their report of the subgroup of 25 patients with SPNET (five of which were pineal), 17 had stage M0 disease and nine underwent GTR (Marec-Berard et al. 2002). Patients were treated adjuvantly with a planned total seven cycles of CBDCA and procarbazine followed by ETOP, CDDP and then VCR and CYCLO with mesna. Only two children completed the full seven cycles. Despite >1/3 of patients demonstrating positive response (CR + PR), the 2-year PFS was 4% and 2-year OS rate was 29% for all patients in this analysis. There was a trend for improved outcomes with extent of surgical resection, although patients with relapse all failed locally. The outcomes of the SPNET patients treated with adjuvant chemotherapy were equally dismal in the SFOP study as in the POG study.
Intensified Chemotherapy
The CCG investigated non-randomized use of a more intensive “eight drugs in 1 day” for children younger than 18 months of age following resection of malignant brain tumors in an attempt to reduce or delay RT (Geyer et al. 1994). The agents used included VCR, BCNU, procarbazine, hydroxyurea, CDDP, Ara-C, CYCLO, and prednisone, and were delivered in 1 day, repeated for a total of eight cycles at 2 weeks for cycle 2 and then monthly thereafter. Of the 82 children enrolled, 19 (23%) had SPNET (11 of these being non-pineal). Six of these 19 had GTR, and 14 had stage M0 disease. In contrast to the other studies, extent of resection was not associated with improved outcomes, suggesting a benefit of this aggressive chemotherapy to treat residual disease. Also contrary to other analyses, the 3-year PFS was significantly improved for patients with non-pineal SPNET versus those with pineal SPNET or cerebellar PNET (55% compared with 0% or 26%, respectively). Similar to the POG and SFOP studies, the recurrences in this group of young patients occurred early (within 6 months), and most frequently were local. Thus deferral of RT after completion of chemotherapy was not considered likely to benefit PFS in these patients. Of note, only a minority of the patients without progressive disease received RT as part of their therapy (Geyer et al. 1994).
As mentioned in the section on 7.7.1.1 above, the POG 9233/34 randomized controlled trials were designed to evaluate standard versus DI chemotherapy for improved EFS in patients less than 3 years old with malignant brain tumors, of which 38 (12%) had SPNET (Strother et al. 2014). These studies employed two different chemotherapy regimens, both including the same agents (CYCLO, VCR, ETOP, and CDDP) administered at slightly different dosages and timing, for a total of 72 weeks. In the combined study, there was no benefit of DI over standard chemotherapy in young patients with SPNET with respect to EFS and OS, and only 20% of these children had disease control without RT.
In an effort to improve outcomes of conventional chemotherapy alone following resection of SPNET, intensification of chemotherapy with autologous stem cell rescue (ASCR) has been investigated. One such multi-institutional trial included 62 children with brain tumors all <6 years of age, 14 of which had SPNET (11 of these being non-pineal), and of these, 50% were <3 years of age at diagnosis (Mason et al. 1998). The children on study received five cycles of CDDP, VCR, ETOP, CYCLO, and mesna over 12–16 weeks, after which they received consolidation with CBDCA, ETOP, and thiotepa followed by ASCR if they were found to have no residual tumor, GTR at time of second-look surgery, or stable residual disease that could be treated with adjuvant RT. Seven of all 14 SPNET patients had GTR, five with STR, and two with “partial” resection. Of the seven who did not have GTR, one died from treatment toxicity, while 2/3 of the remaining children had positive response to the induction therapy. Reported 2-year EFS and OS from diagnosis for all 14 SPNET patients was 43% and 64%, respectively, which was comparable to the 13 children with medulloblastoma included in this study (Mason et al. 1998). Extent of resection tended to correlate with improved outcome. Although the results of this intensive chemotherapy regimen were encouraging, toxicity was not insignificant, with high reported incidence of mucositis (100%), dermatitis (100%), and hearing loss (34%), in addition to the anticipated hematologic toxicity, and there was an 8% incidence of toxic deaths among all the study subjects. Neuropsychological testing also revealed impairment of language and fine-motor functioning in the children who did not receive RT on this trial. The number of children with SPNET requiring RT was not reported, although nearly 50% of all subjects who received the ASCR were able to avoid RT in this study.
The German HIT 2000 study investigated the impact of various adjuvant chemotherapy regimens on outcomes of 17 young children <4 years old with “CNS-PNET” (eight not otherwise specified, eight pineoblastoma, one ependymoblastoma) (Friedrich et al. 2013). For the 11 stage M0 patients, up to five cycles of combination CYCLO, VCR, MTX, CBDCA, and ETOP were given within 8 months, followed by CSI. A shorter 2- to 3-month course of induction CBDCA and ETOP followed by HDCT, both with intrathecal MTX, were administered to three of six positive responders in the M+ group. These three also received CSI after completing systemic therapy. The EFS rate was 24% and OS was 40% at 5 years for all patients, with trend for increased survival noted for those patients receiving the intensified therapy. No difference was detected between patients with pineal and non-pineal tumors. Thirteen patients relapsed, all of which failed during the induction chemotherapy and 11 of which had local recurrence, suggesting limited effectiveness of chemotherapy in treating SPNET.
The Gustave Roussy conducted a more recent study of HDCT with ASCR in 24 high-risk PNET patients over 5 years of age, of which three had SPNET (Dufour et al. 2014). In this trial, following resection, the children received two cycles of induction chemotherapy with CBDCA and ETOP followed by two cycles of high-dose thiotepa with ASCR, after which they were treated with craniospinal irradiation (CSI, to be discussed in more detail in the section 7.7.1.3 below). Nineteen of 21 evaluable patients demonstrated positive response (CR + PR) to HDCT, with expected hematologic and gastrointestinal toxicity. No patients died from the HDCT, and all were able to receive RT. Eight patients relapsed, of which two had SPNET. The 3-year event-free survival (EFS) and OS rates for all evaluable patients were 79% and 82%, respectively. The positive outcomes of this study likely reflect the large percentage of these high-risk patients having cerebellar PNET.
Head Start (HS) I and II are two important multi-institutional, prospective studies investigating benefit of HDCT with ASCR to delay or defer RT for young children with malignant brain tumors. Eligible children <6 years of age were treated on HS I with maximal safe resection followed by HDCT with CDDP, VCR, ETOP and CYCLO plus mesna for five cycles of induction therapy followed by consolidative chemotherapy with CBDCA, ETOP, and thiotepa followed by ASCR. The age was increased to <10 years for subjects on HS II, and patients on this study with M+ disease received high-dose MTX in addition to the HS I regimen above. Children with residual disease after induction underwent second-look surgery as feasible prior to consolidation therapy. Children with unresectable bulky, symptomatic, or progressive disease did not receive consolidation therapy. A total of 43 children with SPNET were treated on the HS trials (Fangusaro et al. 2008). GTR was achieved in 21 of these children. Of the remaining 22 patients with less than GTR, 82% demonstrated positive response to the induction chemotherapy. A total of 32 were able to proceed to consolidation chemotherapy with ASCR. The 5-year EFS and OS for all SPNET patients were 39% and 49%, respectively. Recurrent and/or progressive disease was identified in 25 patients, of which 23 had a local disease component. While the authors report that 15 of 20 survivors were able to avoid CSI and 12 received no RT, it is noteworthy that over 90% of the 25 patients with recurrent disease had local failure. Common adverse effects of this therapy included high-grade hematologic toxicity and hearing impairment, and 5% of the children in this cohort died from this therapy.
Post-RT Chemotherapy
Chemotherapy following RT has also been examined in subjects with SPNET. A multi-institutional study of 53 patients with newly diagnosed PNET, including both supratentorial and cerebellar tumors, demonstrated feasibility of receiving four cycles of HDCT with CDDP, CYCLO, and VCR followed by ASCR after maximal safe resection and CSI (Strother et al. 2001). Forty-nine patients between 3 and 17 years of age were able to complete all four cycles of HDCT, with 2-year PFS of 94% for average-risk and 74% for high-risk patients, based on stage and extent of resection. The proportion of patients with cerebellar PNET was not specified in this work, which may account in part for the very encouraging results compared with those generally observed for SPNET.
The role of maintenance chemotherapy following concurrent VCR and CBDCA with RT in children with newly diagnosed PNET was examined in the phase I/II CCG-99701 study, which was completed in 2012. The results of this study are eagerly awaited.
Chemotherapy for Pineal SPNET
The role of chemotherapy in the treatment of pineal PNET was assessed in the comprehensive analysis of 299 patients with pineoblastoma identified in a literature review by Tate et al. (Tate et al. 2012). In this work, 2-year OS was lowest following treatment with standard postoperative chemotherapy compared with adjuvant RT or chemoradiotherapy (31%, vs. 35% or 60%, respectively). This finding was still observed even after correcting for age, extent of surgical resection, and stage, supporting the limited utility of chemotherapy even in this more favorable subgroup of SPNET patients.
7.7.1.3 Role of RT
Given the overall poor outcomes of patients with SPNET treated with surgery and postoperative chemotherapy alone, RT is considered an essential component of therapy for this aggressive disease.
Standard Fractionated RT
Standard RT regimens for SPNET include CSI followed by a boost to the primary tumor bed, largely extrapolated from studies for cerebellar PNET and retrospective series (Jakacki 2005). One such retrospective study from the University of California San Francisco (UCSF) examined outcomes of 10 children <18 years of age with SPNET treated with RT (McBride et al. 2008). While the RT regimens were quite varied in this review, all five children who received RT early in their treatment course had no evidence of disease, whereas two of five treated with salvage RT recurred, and another three of five who did not receive any RT were deceased at the time of follow-up, with median 6.5 months time to progression in the latter two cohorts. Only half of the patients treated with RT received CSI, ranging from 23.4 to 36 Gy in 1.8 Gy per fraction, five fractions weekly, which was reserved for children >3 years of age at the time of diagnosis. The total dose to the tumor bed ranged from 50.4 to 72 Gy. Given the small patient numbers and range of treatment given in this series, no conclusion could be drawn regarding radiation dose or volume in this disease setting. In contrast, the prospective HIT 88/89 and 91 studies identified radiation dose and target volumes as the only significant prognostic factor for PFS in the subgroup analysis of 63 SPNET patients treated, since those children receiving less than the study-mandated 35 Gy to the neuraxis or 54 Gy to the primary site had 3-year PFS of 7% compared with 49% for those treated with the study regimen (Timmermann et al. 2002).
Due to concerns of adverse sequelae following high-dose CSI, particularly in young patients, Chintagumpala et al. attempted to address the issue of radiation dose in a multi-institutional, prospective pilot study of risk-adapted therapy for SPNET patients between 3–21 years of age (Chintagumpala et al. 2009). A total of 16 subjects with SPNET (seven of these being pineal) were enrolled on this study. Half of the patients were considered average risk (M0 and ≤1.5 cm2 residual tumor) and received 23.4 Gy CSI followed by a boost to a total 55.8 Gy to the tumor bed plus 2-cm margin in once-daily 1.8-Gy fractions. The other eight patients were considered high-risk and received 36–39.6 Gy CSI with same total dose to the boost volume. All patients completed RT, and 14 went on to receive HDCT with CYCLO, CDDP, and VCR followed by ASCR. The 5-year EFS and OS for the average-risk patients were 75% and 88%, respectively, and for the high-risk patients, these outcomes were 60% and 59%, respectively. Outcomes were worse in the patients with pineal tumors compared with non-pineal ones (54% vs. 78% 5-year EFS, and 67% vs. 78% 5-year OS). Four patients relapsed after completing RT: two had some local component and two only had disease outside the primary site. Despite utilizing a reduced CSI dose in the favorable patients, the results of this small study superseded those of other studies using the same or higher dose of RT, suggesting potential benefit of the combined lower CSI dose and more aggressive chemotherapy in these patients (Fig. 7.2).
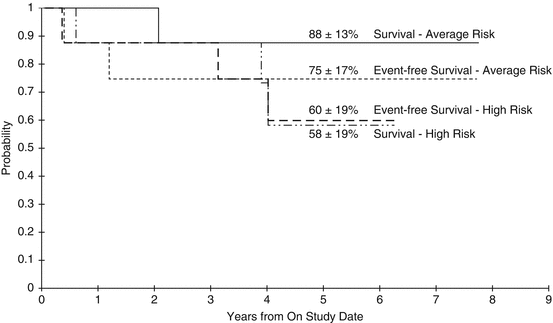

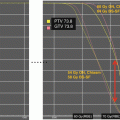
Fig. 7.2
Outcomes of subjects 3–21 years of age with SPNET treated with risk-adapted therapy (from Chintagumpala et al. [2009]). Event-free and overall survival of average-risk and high-risk pediatric patients age 3–21 years treated with risk-adapted chemotherapy and radiation therapy. Note overall improved outcomes compared with historic outcomes
Altered Fractionation RT
In another effort to reduce the late effects associated with standard fractionated RT in this young patient group, Massimino et al. conducted a single-institution study of hyperfractionated accelerated RT (HART) in 15 patients >3 years of age diagnosed with SPNET (three of which were pineal). All patients were treated with surgery followed by moderate dose-intense chemotherapy with MTX, VCR, ETOP, CYCLO, and CBDCA prior to 1.3 Gy per fraction, twice daily, 5 days weekly CSI to 31.2 Gy (for <10 years old) or 39 Gy (for ≥10 years old) and 1.5 Gy per fraction, twice daily, 5 days weekly boost to tumor bed for total dose of 59.6–60 Gy, for a total number of treatment days ranging from 17.5 to 22 (Massimino et al. 2006). The patients then received additional maintenance chemotherapy, initially standard chemotherapy with VCR and CBDCA, changed to HDCT with thiotepa and ASCR due to early relapses in three of five patients in the former cohort. For all patients, 3-year PFS and OS were 54% and 61%. Five of six patients who relapsed had a local component, occurring by a median of 6 months from start of induction chemotherapy. All patients were able to complete HART without significant toxicity, and five of seven patients with residual disease at time of HART had positive response (1 CR + 4 PR) to the radiation. While the authors suggest benefit of HART by delivering higher biologic effective dose with minimal increase in late effect, the observed outcomes were no better than observed in comparable aggressive multimodality therapies, and late effects from treatment were not reported in this study. The logistical issues of delivering twice daily RT to pediatric population (e.g., need for anesthesia) were also not addressed. Consequently, use of HART is not recommended for SPNET.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree