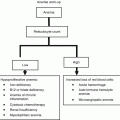
High (emesis risk >90 % without antiemetics)
Carmustine, BCNU
Lomustine
Cisplatin
Mechlorethamine
Cyclophosphamide (>1,500 mg/m2)
Pentostatin
Dacarbazine, DTIC
Procarbazine
Moderate (emesis risk 30–90 % without antiemetics)
Azacytidine
Doxorubicin
Alemtuzumab
Imatinib
Cyclophosphamide (<1,500 mg/m2)
Melphalan i.v.
Cytarabine (>1 g/m2)
Mitoxantrone (>12 mg/m2)
Daunorubicin
Oxaliplatin
Low (emesis risk 10–30 % without antiemetics)
Asparaginase
Methotrexat (>100 mg/m2)
Bortezomib
Mitoxantrone (<12 mg/m2)
Cytarabine (<1 g/m2)
Pegasparaginase
Etoposide i.v.
Teniposide
Gemcitabine
Thiotepa
Minimal (emesis risk <10 % without antiemetics)
Bleomycin
Melphalan p.o.
Busulfan
Mercaptopurine
Chlorambucil
Methotrexat (<100 mg/m2)
Cladribine
Sorafenib
Cytarabine (<100 mg/m2)
Thioguanine
Fludarabine
Vinblastine
Hydroxyurea
Vincristine
α-, β-, γ- Interferone
Vinorelbine
Agents such as Lenalidomide, Thalidomide, Nilotinib, Dasatinib and Ruxolitinib are not classified yet.
Patient Related Risk Factors
Age is an important prognostic factor when dealing with chemotherapy-induced nausea and vomiting. Several studies have shown that complete response from nausea and vomiting are significantly more frequent in patients over 65 years of age [3]. Patient risk factors including further female gender, a history of low alcohol intake, experience of emesis during pregnancy, impaired quality of life and previous experience of chemotherapy are known to increase the risk of nausea and vomiting after chemotherapy.
Co-morbidity
Treatment of elderly cancer patients is often complicated by the circumstance that elderly have a higher incidence of concomitant diseases, e.g. hypertension. Heart diseases are seen in approximately 20 % of elderly patients. This should be considered when treating nausea and vomiting with serotonin receptor antagonists. For example some serotonin receptor antagonists have been shown to lead to prolongation of the QTc interval which increases the risk of cardiac arrhythmia. Diabetes (10–15 % in this population) is frequent in elderly cancer patients and leads to increased risk of hyperglycemia with corticosteroids and increased risk of constipation (side effect of serotonin receptor antagonists, 10–15 %) due to autonomic neuropathy [3].
Antiemetics
5-HT 3 serotonin receptor antagonists (5-HT 3 RAs): The 5-HT3 RAs form the cornerstone of therapy for the control of emesis with chemotherapy agents with moderate to high emetogenic potential. Five 5-HT3-RAs, dolasetron, granisetron, ondansetron, palonosetron and tropisetron are available in Europe. When administering 5-HT3-RAs, several points should be taken into consideration [8–10]:
The lowest fully effective dose for each agent should be used; higher doses do not enhance any aspect of activity because of the receptor saturation.
Oral and intravenous route are equally effective.
No schedule is better than a single dose daily (exception ondansetron) given before chemotherapy.
Neurokinin 1 receptor antagonist (NK 1 RA): Aprepitant-containing regimens have been shown to significantly reduce acute and delayed emesis in patients receiving highly emetogenic chemotherapy (HEC) and moderately emetogenic chemotherapy (MEC), compared with regimens containing a 5-HT3-RA plus dexamethasone only. Aprepitant is available for oral and as fosaprepitant in the intravenous administration form and should be administered as indicated in Table 17.2. Aprepitant is well tolerated. The most common low grade adverse effects reported during clinical trials include headache, anorexia, fatigue, diarrhoea, hiccups and mild transaminase elevation. Aprepitant is metabolised by cytochrome P450 (CYP) 3A4. It is a moderate inhibitor and an inducer of CYP3A4 [11] and has been shown to cause a twofold increase in the area under the plasma concentration curve (AUC) of dexamethasone, which is a sensitive substrate of CYP3A4. Potential interactions with cytotoxic drugs metabolised by CYP3A4 were intensively studied and did not resulted in clinically significant interactions [12]. However, the potential interactions should be carefully selected when treating elderly patients.
Table 17.2
Dose of antiemetics
5-HT 3− receptorantagonist | Route | Recommended dose (once daily) |
Ondansetron | p.o. | 8 mg twice daily |
i.v. | 8 mg (0.15 mg/kg) | |
Granisetron | p.o. | 2 mg |
i.v. | 1 mg (0.01 mg/kg) | |
Tropisetron | p.o. | 5 mg |
i.v. | ||
Palonosetron | p.o. | 0.5 mg |
i.v. | 0.25 mg | |
Steroids | ||
Dexamethasone | p.o./ i.v. | 12 mg (highly emetogenic with aprepitant) |
20 mg w/o aprepitant | ||
8 mg (moderately emetogenic) | ||
NK 1 –receptorantagonist | ||
Aprepitant | p.o. | 125 mg day 1, |
80 mg day 2 + 3 | ||
Fosaprepitant | i.v. | 150 mg day 1 only |
Steroids, dexamethasone: Although not approved as an antiemetic, dexamethasone plays a major role in the prevention of acute and delayed CINV and is an integral component of almost each antiemetic regimen (Table 17.2). Dexamethasone is the most frequently used corticosteroid, although no study reports the superiority of one corticosteroid over another in terms of efficacy. Because of the risk of insomnia, administering corticosteroids in the evening should be avoided. Elderly are often treated with NSAIDs which especially in combination with corticosteroids can lead to gastric bleeding. Elderly patients with a diagnosis of diabetes should be closely monitored, when receiving steroids [3].
Olanzapine: Olanzapine is an atypical neuroleptic drug. By the ASCO (American Society of Clinical Oncology) guidelines olanzapine is recommended as an adjunctive drug and also for patients who experience nausea and vomiting despite optimal antiemetic prophylaxis [5]. This recommendation is supported by the latest study by Navari where olanzapine showed superior efficacy in comparison to metoclopramide in the rescue setting [13].
Dopamine receptor antagonists: Prior to the introduction of 5-HT3 RAs, dopamine-receptor antagonists formed the basis of antiemetic therapy [14]. One of the most frequently used benzamides is metoclopramide. Current guidelines do not recommend metoclopramide for prevention of acute CINV. The current ASCO guidelines recommend that metoclopramide should be reserved for patients intolerant of or refractory to 5-HT3-RAs, dexamethasone and aprepitant [5]. Just recently, the EMA (european medical agency) recommended that metoclopramide should only be prescribed for short-term use (up to 5 days) because of side effects on the nervous system. In addition, the maximum recommended dose in adults has been restricted to 30mg per day.
Benzodiazepines: These drugs can be a useful addition to antiemetic regimens in certain circumstances such as anxiety and risk reduction of anticipatory CINV or in patients with refractory and breakthrough emesis as suggested by all antiemetic guidelines.
Summary of Antiemetic Guideline Based Management of CINV
Three international antiemetic guidelines are available [5, 10, 15]. None of these guidelines have specific recommendations for prophylaxis of CINV in the elderly.
HEC; acute CINV: The guidelines suggest unanimously a combination of 5-HT3 RA, dexamethasone and aprepitant/fosaprepitant within the first 24 h (Table 17.3).
Emesis risk | Acute phase (day 1) | Delayed phase (day 2–5) |
|---|---|---|
High | 5–HT 3 –RA | |
Granisetron; 2 mg p.o./1 mg i.v. | ||
Ondansetron; 16 mg p.o./8 mg i.v. | ||
Palonosetron; 0,5 mg p.o./ 0,25 mg i.v. | ||
Tropisetron; 5 mg p.o./i.v. | ||
Dolasetron; 100 mg p.o. | ||
+ | ||
Steroid | Steroid | |
Dexamethasone; 12 mg p.o./i.v. | Dexamethasone; 8 mg p.o./i.v. day 2–3 (4) | |
+ | + | |
NK–1–RA | NK–1–RA | |
Aprepitant; 125 mg p.o. | Aprepitant; 80 mg p.o. day 2–3 | |
or | ||
Fosaprepitant 150 mg i.v. single-dose | ||
Moderate | 5–HT 3 –RA, Palonosetron preferred | |
0.50 mg p.o. / 0.25 mg i.v. | ||
+ | ||
Steroid | Steroid | |
Dexamethasone; 8 mg p.o/ i.v. | Dexamethasone, 8 mg p.o /i.v. day 2–3 | |
Low | Steroid | None |
Dexamethasone; 8 mg p.o/i.v. | ||
Minimal | None | None |
HEC; delayed CINV: All guidelines suggest the combination of dexamethasone and aprepitant. If fosaprepitant was given on day 1, no further application of fosaprepitant is necessary.
MEC; acute CINV: Patients undergoing MEC regimens should be given a combination of a 5-HT3 RA, preferably palonosetron and the corticosteroid dexamethasone. The ASCO guideline stated that the triple combination (5-HT3 RA, dexamethasone and aprepitant) can be considered in selected patients.
MEC; delayed CINV: Dexamethasone is the preferred agent to use. Nonetheless, when aprepitant was used for the prevention of acute CINV then aprepitant should be used also for the prophylaxis of delayed CINV. 5-HT3 RA can be used as an alternative. However, if palonosetron was the 5-HT3 RA of choice a repeated application is not useful.
Low emetogenic chemotherapy: In patients receiving chemotherapy of low emetic risk, a single agent, such as a low dose of a corticosteroid, is effective. In principle 5-HT3-RAs are not constituents of the prophylactic armamentarium.
Minimal emetogenic chemotherapy: It is suggested by all guidelines that for patients treated with agents of low emetic risk, no antiemetic drug should be routinely administered before chemotherapy.
Chemotherapy Induced Myelosuppression
Evidence suggests that elderly patients are at greater risk of chemotherapy-related toxicities than younger patients. Myelotoxicity is of particular concern, as the haematopoietic reserves of elderly patients have been found to decrease progressively with time [16]. Age-related haematologic changes are reflected by a decline in bone marrow cellularity, an increased risk of anemia, and a declining adaptive immunity.
Neutropenia
The purpose of G-CSF (Granulocyte-Colony Stimulating Factors) is to enable administration of chemotherapy, regardless of the age of the patient. Prophylactic G-CSF may provide elderly patients with the chance of receiving curative doses of chemotherapy and thus, potentially improve survival rates. Additionally, the value of palliative chemotherapy should also be considered in elderly patients and appropriate growth factor prophylaxis facilitates the administration of such therapy.
In a systematic review and meta-analysis of randomised controlled trials comparing primary prophylactic G-CSF with placebo or untreated controls in adults with solid tumors or lymphomas reported a 40 % reduction in febrile neutropenia with the use of G-CSF in patients older than 65 years [17]. The EORTC (European Organisation for Research and Treatment of Cancer) Guidelines, the NCCN (National Comprehensive Cancer Network) Guidelines for Senior Adult Oncology as well as ASCO recommend the prophylactic use of G-CSF in clinical situations where the risk of neutropenia is greater than 20 %. Age older than 65 years was identified as an important patient characteristic that identified individuals for the receipt of prophylactic growth factor treatment. G-CSF are also recommended in older patients receiving curative therapy in the treatment of non-hodgkin lymphomas where maintenance of dose intensity is essential to achieve a good outcome. The use of G-CSF therapy is also appropriate in this group when the risk of neutropenia from individual regimens is less than 20 %, if other patient-related factors suggest a high risk of morbidity and mortality from neutropenia (Table 17.4). Treatment with G-CSF can be used to reduce the duration of neutropenia and the incidence of hospitalizations in these patients. Prophylactic antibiotic use with or without G-CSF has shown similar beneficial effect in some studies but no clear recommendation has been made about their use in elderly patients for prophylactic or secondary use for chemotherapy-induced neutropenia.
Table 17.4
Prophylactic use of G-CSF in the Elderly
Risk of febrile neutropenia from chemotherapy ≥20 % |
Risk of febrile neutropenia from chemotherapy 10–20 % and presence of additional risk factors for infectious complications: |
Previous episode of febrile neutropenia |
Advanced disease |
Heavily pretreated patients |
Presence of cytopenias due to bone marrow involvement |
Malnutrition |
Current infections |
Liver or renal dysfunction |
Multiple comorbidities |
Poor performance status |
The risk of administration of growth factors is minimal, although a slight increase in thrombocytopenia has been reported with the use of GM-CSF (not confirmed for G-CSF).
Thrombocytopenia
Platelet transfusions are the only effective way to manage thrombocytopenia associated with chemotherapy, besides chemotherapy dose reductions or delay of chemotherapy. The appropriate threshold for platelet transfusion during chemotherapy recommended by the ASCO is a platelet count less than 10 × 109/L [18]. These recommended levels may have to be modified in the elderly population who may have other risk factors for bleeding including the use of anticoagulant drugs for thromboembolic events or cerebrovascular or cardiovascular diseases. The thrombopoetin receptor agonists romiplostim and eltrombopag are still under investigation for management for chemotherapy induced thrombocytopenia.
Fatigue
Fatigue is an attendant and very burdening symptom for patients with chronic diseases. Especially the pathophysiology of cancer-related fatigue (CRF) is not well understood. It can last for several months after having stopped a cytostatic therapy. CRF is of course a multifactorial disorder and may come from the disease itself or the application of chemotherapy. Personal factors like age, gender, physical conditions, comorbidities, subacute or chronic inflammation and infection and mental health may play an essential role. CRF highly impacts the quality of life and can be associated with a poor adherence to follow the recommended chemotherapy regimen that would be an indirect risk for therapy failure. Therefore the main diagnostic step to become aware of clinically significant fatigue is to keep this symptom in mind. Unfortunately the treatment or prophylactic options against CRF are limited. Because of the possibility of hazardous drug interactions in elderly patients who generally are treated for several comorbidities the use of stimulating antidepressants is only investigated for episodes of real depression which should be ensured by a specialist. It is already known that phytotherapeutics like ginseng may reduce symptoms from CRF. A recent placebo-controlled phase III trial provides data to support that American ginseng that is in some way different from Asian ginseng reduces general and physical CRF over 8 weeks without side effects when given in a dose of 2,000 mg/day [19]. A beneficial therapeutic approach that was shown by a recent published Cochrane Review of clinical trials [20] is aerobic exercise for individuals with CRF during and after cancer therapy. Apart from that the consequent and generous treatment of chronic anaemia is thought to be important to avoid the worsening of CRF although clinical data providing any thresholds for hemoglobin are missing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree