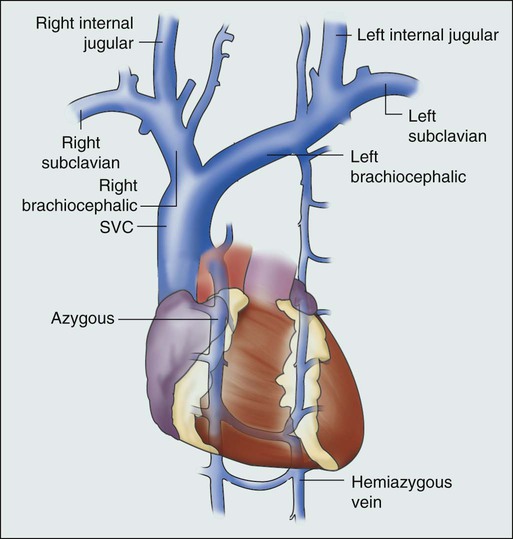
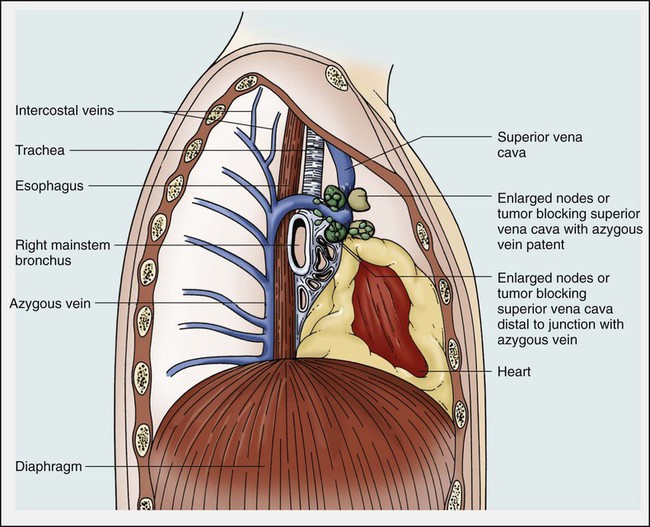
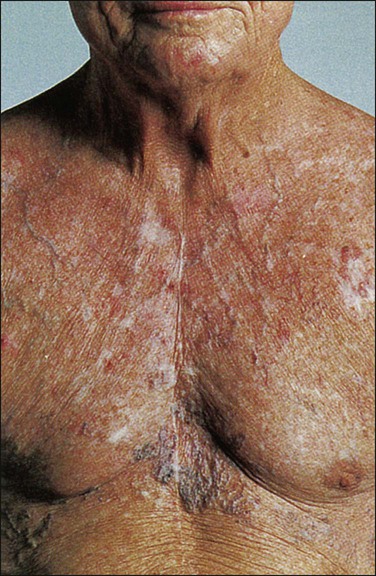
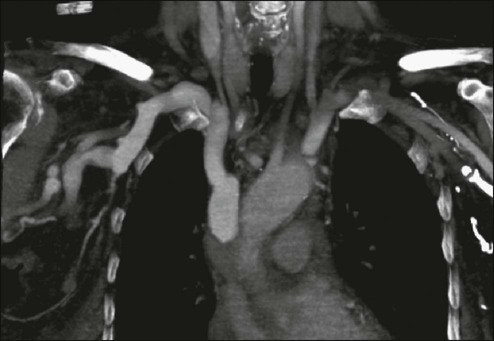
Janessa Laskin, Anthony J. Cmelak, Steven Meranze, John Yee and David H. Johnson • Superior vena cava (SVC) syndrome is usually due to a neoplastic process, predominantly primary lung carcinoma, with a disproportionate number of patients having small cell histology; non-Hodgkin lymphoma and metastatic tumors are the next most common. • SVC syndrome can be iatrogenic; it is sometimes seen as a complication of a central venous line or cardiac surgery. • The junction of the brachiocephalic veins forms the thin-walled, low-pressure SVC, which is subjected to obstruction from a variety of mediastinal components. • External compression often precedes direct tumor invasion or thrombus formation. • The SVC has an extensive collateral network. • Usual symptoms are head “fullness,” dyspnea, cough, and chest pain, typically with insidious onset. • More severe symptoms are infrequent, and life-threatening neurologic symptoms are rare. • Diagnosis is based on clinical findings such as dilated chest veins and facial edema. • A chest radiograph typically shows mediastinal widening; a mass is often seen in the region of the SVC. • Small-dose cavograms can be safely accomplished to define the exact location and routes of collateral flow. • Computed tomography scanning identifies the mass and collateral flow and is the most helpful study to guide treatment. • Treatment of an identified mass before histologic diagnosis is rarely justified unless prior diagnosis is established. • Methods used to define histology are sputum cytology, endobronchial ultrasound with and without bronchoscopy, lymph node biopsy, thoracentesis, percutaneous biopsy, and video-assisted mediastinoscopy or thoracotomy; these techniques are considered quite safe. • Radiation therapy with or without chemotherapy is the preferred treatment in most malignant causes of SVC obstruction, particularly in treatment-sensitive cancers such as small cell lung cancer. • Chemotherapy would be the initial treatment of choice if a definitive diagnosis of lymphoma has been made. • The radiation therapy fractionation schedule depends on tumor histology, stage, prognosis, the patient’s general condition, and whether the obstruction is acute or subacute. • Surgery is usually reserved for select patients with benign causes of obstruction and consists of a bypass procedure. • Percutaneously placed, self-expanding intravascular wire stents provide an option or adjunct to other procedures in the palliative treatment of patients (usually with malignant disease). Obstruction of the superior vena cava (SVC) may occur as an acute or subacute process producing a syndrome with characteristic features including facial edema and plethora, dilation of chest wall and neck veins, mild to moderate respiratory difficulty, and, less commonly, conjunctival edema, central nervous system complaints such as headache, or, more rarely, visual disturbances and signs of altered states of consciousness.1,2 The first recorded description of SVC obstruction (SVCO) occurred in 1757 when William Hunter described the entity in a patient with a syphilitic aortic aneurysm.3 For nearly two centuries thereafter, nonmalignant processes such as aortic aneurysms, syphilitic aortitis, or chronic mediastinitis due to tuberculosis were the predominant etiologic factors.1,2,4 However, these diseases are now quite rare, and cancer has become the leading cause of SVCO primarily because of the rapid increase in the incidence of bronchogenic carcinoma after World War II.1,2,5,6 Although SVCO was once considered a medical emergency, it is now well established that patients with SVCO rarely experience immediate, life-threatening complications.4,7–9 Consequently, in cases in which a diagnosis is not known, it is appropriate to first proceed with a biopsy to establish the underlying cause, because optimal management is dependent on etiology.10 The SVC is formed by the junction of the brachiocephalic veins, which in turn are formed by the joining of the internal jugular and subclavian veins. Thus the SVC represents the major drainage system of venous blood from the head, neck, arms, and upper thorax. The right and left brachiocephalic veins join at about the level of the sternal angle to form the SVC. The SVC descends on the right side of the ascending aorta and empties into the right atrium, with its distal 2 cm lying within the pericardial sac (Fig. 48-1). Because of its mediastinal location and because it is surrounded by several rigid structures including the sternum, trachea, pulmonary artery, right main-stem bronchus, and numerous lymph nodes, the SVC is particularly vulnerable to obstruction. Despite being a relatively large vessel, its thin vascular walls and low intravascular pressure contribute to the ease with which the SVC can be obstructed. SVCO can be caused by external compression due to tumor or by lymph nodes enlarged by inflammation or metastases (Fig. 48-2). SVCO also can be caused by direct tumor invasion or by a thrombus. Secondary thrombus is reported to occur in up to 50% of cases and may contribute to the lack of response to appropriate therapeutic maneuvers. Since the middle part of the twentieth century, cancer has been the principal cause of SVCO, with bronchogenic carcinoma accounting for up to 85% of cases (Table 48-1).1,2,7,11,12 The two most frequent lung cancer histologic types associated with SVCO are small cell and squamous cell carcinoma.1 Although small cell lung cancer (SCLC) accounts for just 15% to 20% of newly diagnosed lung cancers, it is the underlying cause of up to 65% of all cases of SVCO.4,13 The tendency of SCLC to occur centrally within the lung, as well as its high incidence of mediastinal lymph node metastases, most likely accounts for this consistent observation. Although lung cancer is the leading cause of SVCO, the incidence of this syndrome in patients with lung cancer is relatively low. Table 48-1 Causes of Superior Vena Cava Syndrome Non-Hodgkin lymphoma is the second most common cause of SVCO.2,4,14 Perez-Soler and colleagues14 identified 36 cases among 915 patients with lymphoma who were treated at the M.D. Anderson Cancer Center (University of Texas, Houston). SVCO was most commonly observed with diffuse large cell and lymphoblastic lymphomas. The frequency of mediastinal presentations with the latter histologic types may account for this association, because up to 65% of patients with lymphoblastic lymphomas are first seen with a mediastinal mass. The incidence of SVCO in these categories of non-Hodgkin lymphoma is reported to be 7% and 20%, respectively.14 Metastatic cancers account for approximately 5% to 10% of SVCOs (see Table 48-1).1,2,7,11,12 In approximate order of frequency, the most common primary tumor sites are breast cancer, germ cell malignancies, and gastrointestinal cancers. Less common primary sites include sarcomas (including primary sarcomas of the great vessels), transitional cell carcinoma, prostate cancer, and melanomas. However, virtually any cancer capable of metastasizing to the mediastinum can result in SVCO. Nonmalignant causes of SVCO account for up to 5% of cases. An increasingly common benign cause of SVCO is central venous catheter–induced thrombosis, which may occur with cardiac pacemakers, LaVeen shunts, hyperalimentation lines, and Swan-Ganz catheters, as well as those used for chemotherapy administration.5,11,15 SVCO seems to be more common when the tip of the catheter is placed in the left subclavian vein in the upper part of the vena cava.16 Although it has been the subject of many randomized trials, no convincing evidence supports the routine use of thromboprophylactic agents in patients with central venous catheters, as demonstrated by a systemic review and metaanalysis reviewing outcomes with heparin or warfarin; the role for newer anticoagulants is less well defined.17 Additional rare benign causes of SVCO include chronic mediastinitis as a result of histoplasmosis, retrosternal goiters, Nocardia infection, and congestive heart failure.2 In children, SVCO is most frequently related to iatrogenic causes resulting from cardiovascular surgery for congenital heart disease or ventriculoatrial shunts for hydrocephalus. The most common malignant causes of SVCO in children are non-Hodgkin lymphoma, acute lymphoblastic leukemia, Hodgkin disease, neuroblastomas, and yolk sac tumors.18,19 Although the duration of symptoms may range from a few days to several weeks, a majority of patients have symptoms of 4 weeks’ duration or less.1 The physical findings accompanying SVCO such as dilated chest veins and facial edema are diagnostic (Fig. 48-3). Patients frequently complain of a sense of head “fullness,” mild dyspnea, cough, chest pain, and occasionally dysphagia (Table 48-2).1,2,7,12,20 Less frequently, arm edema, stridor, upper body cyanosis, and neurologic symptoms (e.g., headaches or lethargy) may occur. All symptoms may be aggravated by positional changes, particularly those associated with lowering of the head—for example, bending to put on shoes. Table 48-2 Signs and Symptoms of Superior Vena Cava Obstruction Although the clinical pattern is readily described and recognized, it was only recently that a grading or classification system was proposed based on the Common Terminology Criteria for Adverse Events that is widely used in oncology clinical trials.21 If this system were to be widely adopted, it would help streamline data collection. The prospect of catastrophic neurologic events has led to the characterization of SVCO as an “oncologic emergency.”13,19 However, recent reviews have clearly demonstrated that although the clinical presentation can be dramatic, life-threatening neurologic symptoms such as seizures, syncope, or coma rarely occur.4,5,8,10 A standard chest radiograph is the first radiographic procedure performed when SVCO is suspected, with the most common abnormality being mediastinal widening. Typically, a mass is found in the superior mediastinum, right hilum or perihilar region, or right upper lobe; however, normal findings of a chest radiograph do not rule out the diagnosis of SVCO.2 A contrast-enhanced chest computed tomography (CT) scan provides visualization of extravascular and intravascular tumor, as well as thrombus formation within the SVC, and also demonstrates collateral flow. A CT diagnosis depends on diminished or absent contrast opacification of central venous structures such as the innominate vein or the SVC inferior to the obstruction, and opacification of collateral venous routes, especially anterior subcutaneous collateral veins (Fig. 48-4).22 Because dilution of the contrast medium by unopacified blood or the displacement of blood by laminar flow may simulate an intraluminal filling defect, both criteria must be present for the diagnosis of SVCO to be made.22 The anatomy defined by a CT scan may help to guide a fine-needle aspiration biopsy or another diagnostic procedure if a histologic diagnosis has not been previously established. The current-generation helical CT scans also have been used to diagnose SVCO with results that correlate well with regular contrast CT scans. In addition, helical scans can potentially reveal more information regarding the site and extent of disease and the collateral pathways involved, as well as define soft-tissue abnormalities. In the absence of a known cause of SVCO, every effort should be made to obtain a histologic diagnosis before the initiation of any therapy for two reasons.4,5,8,10 First, a definitive diagnosis is necessary to plan therapy, and second, even a brief course of radiation therapy before establishing a diagnosis can make histologic diagnosis difficult or even impossible.10,23 The least invasive diagnostic technique should be performed initially, followed by more invasive procedures as necessary (Box 48-1). Procedures commonly used to establish a tissue diagnosis include sputum cytology, bronchoscopy with or without endobronchial ultrasound-guided biopsies of the lung or mediastinum, peripheral or mediastinal lymph node biopsy, and video-assisted thoracotomy and mediastinoscopy, although the diagnosis is sometimes obtained through other means such as thoracentesis and percutaneous lung biopsy with or without ultrasound guidance.4,24–26 The complication rate of invasive procedures in the face of SVCO is fairly modest. Schraufnagel and associates8 reviewed the outcome of 93 invasive procedures in 62 patients with diagnostic problems, and none of the procedures, including bronchoscopies and mediastinoscopies, was associated with a fatal outcome. Yellin and coworkers12 performed 27 invasive diagnostic procedures in 63 patients with SVCO. No mortality or major bleeding episodes were observed, and diagnostic material was obtained in 89% of patients. Ahmann4 reported that complications of bronchoscopy and lymph node biopsies are virtually nonexistent and that contrast studies, such as nuclear medicine venography, are remarkably safe in the presence of SVCO. Of the various invasive procedures used to obtain tissue in patients with SVCO, mediastinoscopy seems to be the most risky. However, even this procedure has a relatively low complication rate. Mineo and colleagues24
Superior Vena Cava Syndrome
Introduction
Anatomy and Pathophysiology
Etiology
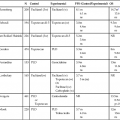
Parish et al.2
Yellin et al.12
Rice et al.11
Armstrong et al.7
Bell et al.1
Total No. of patients
86
63
78
125
159
Lung cancer
45
30
36
99
129
Non–small cell lung cancer
33
26
19
57
64
Small cell lung cancer
12
4
17
42
65
Lymphoma
8
13
6
18
3
Metastases
12
4
2
8
4
Thymoma/thyroid
2
4
–
–
–
Benign
19
11
31
–
2
Biopsy not done
–
–
–
–
21
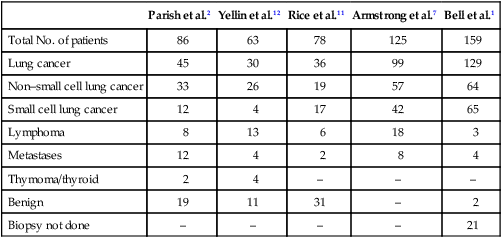
Clinical Features
Total Patients
(N = 489)
Frequency (%)
Edema/suffusion
290
59
Dyspnea
254
52
Cough
100
20
Pain
60
12
Dysphagia
30
6
Syncope
12
2
Arm edema
104
21
Stridor
2
0.4
Neurologic
2
0.4
Hemoptysis
5
1
Radiographic Findings and Diagnostic Studies
Imaging Studies
Diagnostic Approach
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Oncohema Key
Fastest Oncology & Hematology Insight Engine