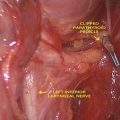
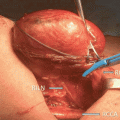
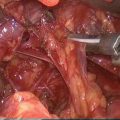
Fig. 17.1
Total numbers of documents on EBSLN published by year (1935–2013)
IONM is increasingly performed in endocrine surgery due to a significant improvement of the technical aspects, devices (Fig. 17.2), and evidences evolutions (Table 17.1). The recent international standards guideline statement on EBSLN monitoring highlighted in detail the advantages of intraoperative neuromonitoring (IONM) for accurate, early, and definitive identification and preservation of the EBSLN during thyroidectomy [5]. The International Neural Monitoring Study Group (INMSG) suggests EBSLN monitoring in thyroidectomy [5]. Recent studies enhanced the utility of IONM research for precise EBSLN neurophysiologic data [43]. An unequivocal definition of EMG data from EBSLN monitoring is a prerequisite for correct definition of preserved nerve function, nerve stress and injury, recovery, and prognostication.
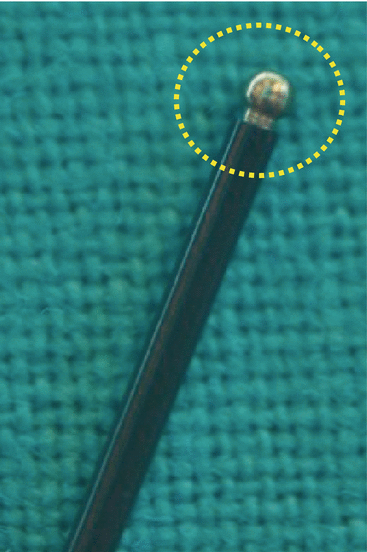

Fig. 17.2
Atraumatic ball tip intraoperative nerve-monitoring probe stimulator for early EBSLN identification and confirmation
Table 17.1
Reasons for increase usage of IONM
Noninvasive devices (endotracheal tube-based monitoring systems) |
User-friendly systems |
Randomized trials |
Guidelines and standardization |
Training courses |
Medicolegal issues |
Research |
Societies recommendations |
Commercial effort |
This chapter is a review of the evidence-based literature and the cumulative experience of the authors with EBSLN monitoring technique. EBSLN monitoring is our routine practice since 2007 [15] for both conventional and endoscopic procedures.
17.2 EBSLN Surgical Anatomy
EBSLN is one of the two main branches of the superior laryngeal nerve (SLN). It innervates the cricothyroid muscle (CTM) and the inferior pharyngeal constrictor muscle. Its anatomical position exposes it to damage risk during the dissection of the upper thyroid pole in the course of thyroidectomy procedures.
The SLN is the only nervous structure whose embryological development originates from the fourth pharyngeal arch. The thyroid, cricoid, arytenoid, corniculate, and cuneiform cartilages of the larynx are formed by the cartilaginous components of the fourth and sixth pharyngeal arches fused together. From the fourth arch develops also the cricothyroid muscle, the levator palatini muscle, and the constrictor of the pharynx, innervated by the different branches of the SLN [50].
The trunk of the SLN arises just caudal to the jugular foramen of the basicranium, branching from the vagus nerve or the nodose ganglion. It runs behind the carotid arteries descending in an anterior and medial direction toward the larynx [21, 22, 31]. The nerve splits into two branches nearly at the level of the superior cornus of the hyoid bone, averaging at 15 mm from its origin. The bifurcation often lies between the internal and the external carotid arteries. Both the branches exit from the carotid sheath about 20 mm below the bifurcation of the common carotid artery [25, 31, 40].
The internal branch of the superior laryngeal nerve (IBSLN) is always in a medial position relative to the lingual and facial arteries. It runs mainly parallel and medially to the superior laryngeal artery. It stays next to the pharyngeal wall, turns medially, caudal to the greater horn of the hyoid bone, passes behind the thyrohyoid muscle, and pierces the thyrohyoid membrane to get into the larynx [23].
EBSLN has many anatomical variants and knowing the variations in its course is fundamental for its identification during surgery. The diameter of the nerve is about 0.2 mm [31]. Its vascularization is supplied by the cricothyroid artery, branch of the superior thyroid artery (STA) [41]. After its branching, it crosses the medial border of the internal carotid artery (75 %) or runs posteriorly to the common carotid artery, lying medial to the cervical sympathetic ganglion [41].
Four different classifications for the position of the nerve have been proposed. Each of this classification uses different landmarks. Cernea classification [9] is the most commonly cited in the past and recent literature and it is simple and easy to be applied in the clinical practice.
17.2.1 Classifications
Cernea et al. [9] and Kierner et al. [33] describe the nerve path in relation to the STA, Friedman et al. [19] use as landmark the inferior constrictor muscle (ICM), and Selvan et al. [53] analyze the relationship between the entries of the nerve into the cricothyroid muscle, the superior thyroid vessels (STV), and the cricoid cartilage. Table 17.2 summarizes these classifications.
Table 17.2
EBSLN classifications
Cernea et al. [9] |
Type 1 → the nerve crosses the STA more than 1 cm above the margin of the superior thyroid pole (68 % in case of small goiter; 23 % in case of large goiter) |
Type 2A → the nerve crosses the STA <1 cm above the margin of the superior thyroid pole (18 % in case of small goiter; 15 % in case of large goiter) |
Type 2B → the nerve crosses the superior thyroid pedicle below the margin of the superior thyroid pole (14 % in case of small goiter; 54 % in case of large goiter) |
Kierner et al. [33] |
Type 1 → the nerve crosses the STA more than 1 cm above the margin of the superior thyroid pole |
Type 2 → the nerve crosses the STA <1 cm above the margin of the superior thyroid pole |
Type 3 → the nerve crosses the superior thyroid pedicle below the margin of the superior thyroid pole |
Type 4 → the nerve crosses the STA immediately above the margin of the superior thyroid pole |
Friedman et al. [19] |
Type 1 → the nerve runs superficially or laterally to the inferior constrictor muscle for its whole course until it terminates in the cricothyroid muscle |
Type 2 → the nerve penetrates the inferior constrictor muscle in its lower portion |
Type 3 → the nerve goes under the superior fibers of the inferior constrictor muscle covered for the whole course |
Selvan et al. [53] |
Type 1a → the nerve lies 1 cm before the entry of the vessels into the gland or anterior/between the branches of the superior thyroid vessels and distant 3 cm from the cricoid cartilage (9 %) |
Type 1b → the nerve lies 1 cm before the entry of the superior thyroid vessels into the gland but posterior to the vessels. The entry point is close to the anterior insertion of the cricothyroid muscle on the cricoid cartilage (3 %) |
Type 2 → the nerve lies within 1–3 cm of the entry of the vessels into the gland or within 3–5 cm from the cricoid cartilage (68 %) |
Type 3 → the nerve lies between 3 and 5 cm of the entry of the vessels into the gland or more than 5 cm from the cricoid cartilage (20 %) |
The Cernea et al.’s [9] classification divides EBSLN into type 1, that is, the nerve crosses the STA more than 1 cm above the margin of the superior thyroid pole (68 % in case of small goiter; 23 % in case of large goiter); type 2A, that is, the nerve crosses the STA <1 cm above the margin of the superior thyroid pole (18 % in case of small goiter; 15 % in case of large goiter); and type 2B, that is, when the nerve crosses the superior thyroid pedicle below the margin of the superior thyroid pole (14 % in case of small goiter; 54 % in case of large goiter).
Cernea 2A and 2B types are particularly frequent (15–55 %) and prone to be injured during the upper thyroid pedicle dissection and ligation.
Cernea classification is nowadays the most commonly used EBSLN surgical classification in the clinical practice and research, for its simplicity and ease of use.
17.2.2 The Sternothyroid–Laryngeal Triangle
Knowing this anatomical space, otherwise called Jolles space, is useful to find the EBSLN. The triangular region has the base at the bottom, represented by the upper edge of the superior thyroid pole; the medial margin is composed of the thyroid and cricoid cartilages, the pharyngeal constrictors and cricothyroid muscle, and the retracted sternothyroid muscle which normally lies above laterally. In the medial portion of the area, it is usually possible to find the EBSLN. The STV are located laterally and the STA between the vein and the nerve [42].
17.2.3 EBSLN Anastomoses
Another important aspect about the EBSLN is the presence of anastomoses between its trunk and the other nerves, particularly with the IBSLN and the recurrent laryngeal nerve (RLN). The connection between IBSLN and EBSLN runs through the thyroid foramen (an opening inside the thyroid cartilage) when present, often followed by the blood vessels [52]. The human communicating nerve (HCN) [61], also called pyriform nerve [51] or cricothyroid connection branch [38], is the anastomosis which connects the EBSLN to the RLN in one or both sides. Its position between these nerves suggests that it could be the nerve of the fifth branchial arch [51, 61]. It starts from the medial surface of the cricothyroid muscle and enters into the lateral surface of the thyroarytenoid (TA) muscle. Sometimes it is double branched, but more frequently it is a single trunk [38].
Depending on the position where the human communicating nerve joins the RLN, four variants of anastomoses have been described [38]:
Type A → the HCN enters the RLN in correspondence to the inferior branch to the posterior cricoarytenoid muscle
Type B → the HCN enters the RLN where the arytenoid branch originates
Type C → the HCN enters the RLN where the lateral cricoarytenoid branch starts
Type D → the HCN enters the last RLN into the branch to the thyroarytenoid (TA) muscle
This nerve is composed of sensory fibers, passing into the TA muscle to terminate near the cricoarytenoid joint in the subglottic mucosa, and motor fibers joining the RLN or entering the TA muscle directly [61].
17.3 Incidence of EBSLN Injury
The prevalence of EBSLN damage is hard to assess because, at this stage, there is too much intertrial methodological heterogeneity. Since vocal examination underestimates the incidence of neural damages and laryngeal postoperative exam can have inconsistent findings as well, CTM EMG is the only reliable way to diagnose EBSLN injury. Hence, the prevalence of such event ranges from 0 % to nearly 60 %. For all those reasons, EBSLN damage is considered the most commonly underestimated postoperative event [3, 5, 29, 32, 36, 46, 47]. However, higher intraoperative attention is given to the EBSLN (in particular during the dissection of the superior thyroid pole); the lower nerve damage rate is also reported. What is more, in recent years the increased use of IONM improved the neural detection rate and decreased the neural impairment rate [4, 5, 9, 18, 30].
Cernea et al., showed an incidence between 12 % and almost 30 % when the EBSLN was not identified during surgery [9, 10]. Jansson et al. reported a temporary EBSLN injury rate of almost 60 % and a permanent injury rate of almost 4 % when no EBSLN identification was achieved [29]. Additionally, a randomized trial showed that the distal ligation of the superior thyroid vascular branches without neural visual identification, if performed by experienced surgeons, seems to be a safe procedure, but there is a temporary EBSLN lesion prevalence superior to nerve identification technique (0.5 % vs. 0.8 %), and, of note, these authors did not report EMG definition of CTM function [6].
17.4 Diagnosis and Treatment of the EBSLN Injury
Clinical or endoscopic findings cannot accurately diagnose EBSLN dysfunctions, because they are often subclinical and widely variable (from loss of ability to produce high-pitched sounds to a deeper voice acquisition, from singing voice impairment to vocal tightness/weakness, or requiring extra effort in speaking) [5, 8, 48, 49, 55]. These symptoms may significantly affect the quality of life [12, 32]. Via the functional voice assessment, shortening of the maximum phonation time and lowering of the high tones and reduction of the vocal range can be detected. The effect of cricothyroid muscle dysfunction has controversial and variable effects at laryngoscopy exam [1, 5, 42, 56, 57, 60].
Since the clinical findings are often minimal and the laryngeal findings are heterogeneous, debatable, and not conclusive, EMG of the CTM (via percutaneous electrode placement into the muscle) is the most reliable way to detect EBSLN dysfunction (details of the technique are described in the guidelines) [5, 9, 59]. After temporary EBSLN damage (in case of paresis or unilateral paralysis), vocal quality improvement can follow; moreover residual muscular function can be implemented via vocal exercise. Notably, at this stage, when EBSLN damage is bilateral and complete, there is no effective way to restore dynamic pitch range [5]. Promising data were presented by El-Kashlan et al., who successfully performed selective cricothyroid muscle reinnervation (in a small sample with high vagal lesions). After reconstruction, all of these patients showed electromyographic and voice quality improvement [18].
IONM has been proposed for EBSLN test for postoperative prognostication. IONM is useful for intraoperative detection of nerve injury. Intraoperative electrical EBSLN testing at the end of the operation “S2” (Table 17.3) can serve for postoperative prognostication of the EBSLN function [5, 45]. At the end of the surgical operation, a positive cricothyroid twitch after stimulation of the EBSLN is a good evidence for functional EBSLN preservation, with a related low risk of intraoperative neural damage [5, 45]. It is mandatory to investigate the most cranial neural segment, above the region of the superior pole managed during surgery. Moreover, it is important to have accurate knowledge of the normal laryngeal and cricothyroid muscle anatomy to accurately assess the cricothyroid muscle twitch [5, 45].
Table 17.3
Standardization of RLN and EBSLN monitoring
1. L1 Preoperative laryngoscopy |
2. V1 Test vagus nerve before identification of RLN |
3. R1 Test RLN when it was identified at the tracheoesophageal groove |
4. S1 EBSLN stimulation at identification |
5. S2 EBSLN stimulation after STA ligation |
6. R2 Test RLN after it was completely dissected from Berry’s ligament |
7. V2 Test vagus nerve after complete hemostasis |
8. L2 Postoperative laryngoscopy |
17.5 EBSLN Monitoring Technique
Standards for EBSLN management during thyroid surgery are detail knowledge of its anatomy, experience, training, visual identification, and exposure. EBSLN monitoring principles are similar to those of recurrent laryngeal nerve (RLN) monitoring, that is, IONM is an adjunct to meticulous technique and useful for EBSLN identification, confirmation, monitoring, functional verification, and exclusion of EBSLN proximity during dissection. Multiple surgical techniques have been reported to reduce the risk of injury to the EBSLN [5]: (1) ligation of the superior thyroid vascular branches under direct vision on the thyroid capsule without visual neural identification [6], (2) visual neural detection before superior thyroid pole vessel ligation [1], and (3) the use of either a nerve stimulator or intraoperative neuromonitoring for mapping and confirmation of the EBSLN identification [2, 4, 9, 10, 15, 20, 28, 30, 35].
17.5.1 Equipment and Setup
RLN and EBSLN share certain monitoring standards of equipment setup, endotracheal tube placement, anesthesia, and correct tube positioning verification tests [45]. Monitoring is set with event threshold of 100 mcV (150 mcV usually for RLN) and pulsatile stimulus duration of 100 mcs at 4 Hz [5]. We commonly use the intermitted IONM manual stimulator probe as dissecting instrument, disposable or reusable. We prefer the ball tip stimulator probe which is atraumatic during dissection (Fig. 17.2). On the other hand, stimulating electrodes may be monopolar or bipolar. Bipolar stimulator may be preferred to monopolar one for EBSLN monitoring due to its small nerve diameter, less stimulation artifacts, convergence, and precision of stimulation (Fig 17.3). Bipolar stimulator may provide the potential greater sensitivity [5]. If a bipolar device is used, the precise orientation of the anode and cathode stimulating electrodes placed on the nerve is of crucial importance. The bipolar probe may not be the best option for nerve mapping since the stimulation is more focal in comparison with the monopolar probe, which provides more diffuse current spread and may facilitate the mapping of a wider region [5]. The stimulator probe should be set at 1–2/3 mA. Stimulation is set with 2–3 mA for nerve identification, mapping, and dissection; with 1 mA for nerve confirmation, monitoring, S1 and S2 stimulation (Table 17.3). In particular, for confirmation of visually identified EBSLN, a 1 mA current should be preferred but higher values (up to 2 mA) should be used for EBSLN mapping. Multiple and heterogeneous different nerve-monitoring formats have been studied but for safety and simplicity reasons; endotracheal tube-based surface electrode systems are the most commonly used monitoring system [45]. De facto, endotracheal tube-based systems with a graphic monitor documentation are preferred for neural monitoring. New audio and graphic monitor IONM equipment must be preferred today to only audio monitors due to the possibility of print documentation, amplitude and latency quantification, storage/record, differentiation between signal and artifact, forensic issues, research, and justifications of surgical deliberations. In fact, audio-only systems lack EMG response stimulation assessment (threshold, amplitude, latency, and waveform shape) [5, 45]. We routinely adopt EMG electrode surface endotracheal tube. Endotracheal EMG tube electrodes gain more popularity recently due to its availability, safety, noninvasive nature, ease of setup, ease of use, and the capacity to derive larger areas of evoked muscle potentials as for EBSLN monitoring. Finally, the SLN is the first branch of the vagal nerve after skull base exit; thus continuous monitoring (C-IONM) probe electrode cannot monitor EBSLN; however C-IONM may provide simultaneous and permanent monitoring of RLN during upper pole dissection.
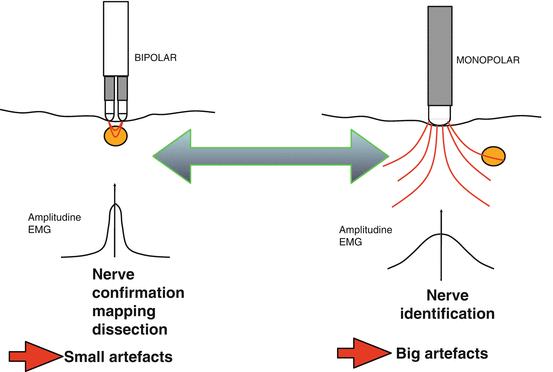

Fig. 17.3
Intraoperative nerve-monitoring probes: bipolar vs. monopolar. Bipolar stimulator may be preferred to monopolar one for EBSLN monitoring due to its small nerve diameter, less stimulation artifacts, convergence, and precision of stimulation
An accurate surgical technique must be adopted to spare the cricothyroid muscle and the EBSLN. The EBSLN course must be visually identified and monitored in order to avoid neural damage when approaching the superior pole by identification of its course. For this reason, a perfect anatomical knowledge and the classification of the different EBSLN variations are mandatory [5]. In the avascular space between the superior pole and the cricothyroid muscle, a blunt dissection of the superior pole should be performed to obtain clear exposure of the sternothyroid–laryngeal triangle where the EBSLN is located (Fig. 17.4). In the vast majority of cases, there is no need for transverse division of the strap muscles if the thyroid has a normal size or it is only slightly enlarged. However, in cases of large masses or if a short neck is present, a partial division of the sternothyroid muscle may improve the surgical access. Moreover, a better exposure of the sternothyroid–laryngeal triangle can be achieved via gentle latero-caudal traction of the thyroid lobe [5]. The transverse division of the cranial portion of the sternothyroid muscle should be performed with caution due to possible proximity of EBSLN. Previous stimulation/mapping is suggested to exclude EBSLN proximity with a stimulation set between 2 and 3 mA.
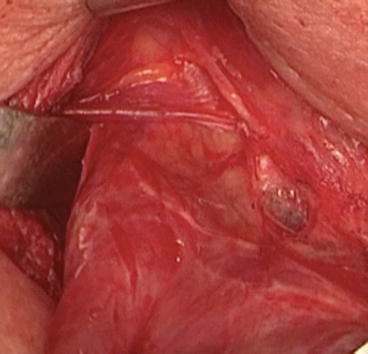

Fig. 17.4
The sternothyroid–laryngeal triangle, otherwise called Jolles space, is useful to find the EBSLN. The triangular region has the base at the bottom, represented by the upper edge of the superior thyroid pole; the medial margin is composed of the thyroid and cricoid cartilages, the pharyngeal constrictors and cricothyroid muscle, and the retracted sternothyroid muscle which normally lies above laterally. In the medial portion of the area, it is usually possible to find the EBSLN
Via a blunt dissection, the superior thyroid vessels should be identified, and individual branches exposed [5]. Notably, transverse division of sternothyroid muscle (its superior edge) and a latero-caudal traction of the superior thyroid pole, followed by blunt dissection within the avascular plane of sternothyroid–laryngeal triangle, provide improved EBSLN exposure. The nerve is usually descending parallel to the superior thyroid artery and it is located on the inferior constrictor muscle before its termination within the cricothyroid muscle [5].
It is vital to visually search for the nerve, but of note, almost 20 % of EBSLNs cannot be visually identified because of their subfascial/intramuscular course [19, 34]. Selvan et al. have shown that in many cases, non-neural tissue can be misidentified for the EBSLN [53].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree