Thyroid-related causes
Systemic causes
Hashimoto’s thyroiditis
Diabetes mellitus
Iodine deficiency
Celiac disease
Overtreatment of Graves’ disease
Chronic renal failure
Transient neonatal hyperthyrotropinemia
Syndromes (Turner, Down …)
Variations in genes of TSH/thyroid hormone pathway
Overweight/obesity
X-ray treatment of the head-neck region
Medications (carbamazepine,valproic acid, domperidone …)
Nonthyroidal illnesses include diabetes mellitus, cystic fibrosis, celiac disease, chronic renal failure, and many syndromes, such as Turner, Down, Klinefelter, and Williams syndrome [10].
A condition often associated with SH is overweight/obesity, even if a clear cause-effect relationship has not been established yet. Whether an elevated TSH value in childhood obesity is adaptive, increasing energy expenditure rate in order to prevent further weight gain, or indicates subclinical hypothyroidism or thyroid hormone resistance is still controversial [11, 12].
A condition of SH may also be related to the use of some medications, in particular carbamazepine, valproic acid, or domperidone, and to X-ray treatment to the head and neck for malignant diseases [13].
SH may be the outcome of transient neonatal hyperthyrotropinemia [14]. The prevalence of SH in newborns considered false positive for congenital hypothyroidism (i.e., with high TSH at birth and with normal fT4 and normal or slightly elevated TSH at the confirmatory examination) was reported to be about 50 % in infancy and early childhood and decreases with age, being still >30 % in late childhood [15].
Mutations in genes encoding proteins involved in the TSH pathway have been demonstrated to be responsible for SH [16]. TSH exerts its activity by binding to the extracellular domain of TSH receptor (TSH-R), a G protein-coupled seven-transmembrane domain receptor located in the basolateral membrane of thyroid follicular cells. Some loss of function mutations, which may interfere with the normal receptor function leading to SH, have been identified in the TSH-R gene [17–21]. Moreover, polymorphisms or mutations in genes of the thyroid hormone pathway such as dual oxidase 2, phosphodiesterase 8B, iodothyronine deiodinases, and thyroperoxidase may be responsible of the elevation of TSH level with normal fT4 values [22–25].
17.4 Clinical Manifestations
The clinical presentation varies widely, ranging from no manifestations to clear signs or symptoms of hypothyroidism. The most common clinical sign is goiter, reported to be twice as prevalent as observed in the general population [10]. The abnormalities most frequently associated in the pediatric population are weight gain [16], increased cholesterol levels, impaired growth velocity, anemia, sleepiness, weakness, and impaired psychomotor and cognitive development [8, 26].
However, a recent review analyzing the natural history of SH in pediatric subjects reported in the studies included normal height, BMI, and the age of puberty onset and no clinical manifestations of hypothyroidism, regardless of the evolution of thyroid function [27]. And a recent Italian study analyzing growth and intellectual parameters in a cohort of children with persistent idiopathic SH demonstrated no alterations in growth, bone maturation, BMI status, and cognitive functions despite persistently elevated TSH values [28]. Therefore, thyroid hormones involved in growth and neurocognitive development seem to work properly, regardless of the persistence of elevated TSH levels, in the absence of any replacement therapy.
17.5 Diagnosis
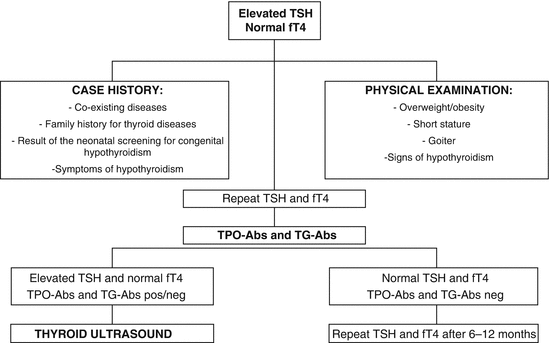
The laboratory detection of a TSH value above the normal upper limit in the presence of normal thyroid hormone levels should raise the suspicion of SH. As serum TSH concentration varies over time even in healthy subjects, an abnormally elevated value may be an occasional finding. Therefore, the measurement of serum TSH and fT4 should be repeated within 3–4 months. A careful case history should be collected, including coexisting diseases, family history for thyroid diseases, and result of the neonatal screening for congenital hypothyroidism. Symptoms of hypothyroidism, such as constipation, poor memory, fatigue, slow thinking, anxiety, depression, muscle weakness, and cold intolerance, should be investigated. An accurate physical examination should be performed in search of signs of thyroid failure, such as overweight/obesity, short stature or impaired growth velocity, hoarseness, and thinning hair. In particular, thyroid region should be inspected and palpated in order to detect goiter. If the isolated TSH elevation is confirmed, antithyroglobulin antibodies (TG-Abs) and antithyroperoxidase antibodies (TPO-Abs) titer should be evaluated and thyroid ultrasonography performed.
17.6 Natural History
The natural evolution of SH may be the persistence of a condition of SH (i.e., normal thyroid hormone levels with stably elevated or even raised TSH levels), the reversion to euthyroidism (i.e., normalization of TSH values with normal thyroid hormone levels), or the progression to overt hypothyroidism (high TSH levels with reduced thyroid hormone levels).
In adult populations, SH was reported to progress toward overt hypothyroidism (reduced circulating thyroid hormones) in proportions ranging from <1 up to 20 % according to different studies [1, 3, 4]. Higher rates of progression to hypothyroidism were reported in patients with higher baseline serum TSH concentration, elevated antithyroid autoantibodies, and a higher degree of hypoechogenicity at thyroid ultrasound [6, 29].
Studies regarding the natural history of SH and its consequences in childhood are scanty [27, 30]. According to the available studies [15, 31–38], SH in children and adolescents seems to be a benign and remitting process with a low risk of evolution toward overt hypothyroidism. Indeed, most of the subjects analyzed in these studies reverted to euthyroidism or remained SH, sometimes with an increase in TSH values. The rate of development of an overt hypothyroidism ranged between 0 and 28.8 %; only one study [36] showed the evolution toward overt hypothyroidism in half of the eight included children.
The initial presence of goiter and elevated TG-Abs, the presence of celiac disease, and a progressive increase in TPO-Abs and TSH value may be predictive of progression toward thyroid failure [38, 39]. Moreover, an initial TSH higher than 7.5 mIU/l and the female gender are predictive factors for a sustained elevated TSH [37].
Notably, a high persistence of SH in “false-positive” children ‘at neonatal screening for congenital hypothyroidism was reported in one study, even if none of the children developed an overt hypothyroidism in the follow-up period [15]. Studying thyroid morphology, the presence of hemiagenesis, hypoplasia of one lobe, or goiter were detected in half of these children and mutations in TPO and TSH-R genes found in two children. Therefore, a mild hyperthyrotropinemia at neonatal screening may be suggestive of congenital anatomic or functional anomalies of the thyroid gland and may be followed by persistent SH later on in childhood.
17.7 Treatment Options
In the decision to treat or not a condition of SH in children, pediatricians should consider on the one hand the risk of progression to overt hypothyroidism and on the other hand the systemic consequences of leaving the hyperthyrotropinemia untreated. In adults, SH has been associated with an increased risk for cardiovascular diseases [40–43] and biochemical abnormalities, including elevated LDL cholesterol, increased serum prolactin concentrations, and a negative influence on the hemostatic profile [44–47]. However, clinicians should likewise consider the risks of an overtreatment, since an overtreated SH could bring on subclinical hyperthyroidism, which has been reported to be responsible for significant bone loss in postmenopausal women [48] and atrial fibrillation in older patients [49]. Moreover, potential benefits of higher TSH levels such as longevity and lower all-cause mortality have been suggested by recent studies [50, 51].
Indeed, the decision regarding the use of levothyroxine (L-T4) replacement therapy for SH is still a matter of debate. In 2004 and 2005, two expert panels came to different conclusions about the management of SH in adults. One expert panel [1] concluded that patients with normal fT4 and TSH >10 mIU/l may be treated, whereas it advised follow-up of subjects with TSH in the range of 4.5–10.0 mIU/l because of insufficient evidence to support treatment in these latter patients and because of the concern of overtreatment. On the other hand, a consensus statement jointly published by the American Association of Clinical Endocrinologists, the American Thyroid Association, and The Endocrine Society [52] concluded that treatment of SH patients with TSH levels of 4.5–10 mIU/l was appropriate, as the lack of evidence of benefit does not necessarily mean a lack of benefit.
Both panels did not address the issue of SH in the pediatric population. Very few studies have examined the effects of L-T4 replacement therapy in young people with SH [27, 30]. L-T4 therapy in children with SH may be beneficial in the presence of short stature and impaired growth velocity [53, 54] and may be effective in reducing thyroid volume [55]. No effects of L-T4 on neuropsychological functions of children with SH have been reported [26].
Hyperthyroidism due to an overtreatment of SH should be considered an infrequent condition in the pediatric age-group.
According to the available evidence, in children with SH L-T4 treatment should be indicated when TSH >10 mIU/l or when TSH 4.5–10.0 mIU/l if in the presence of clinical signs or symptoms of impaired thyroid function, goiter, or coexisting conditions such as syndromes (Down, Turner, etc.) or autoimmune diseases (diabetes mellitus, celiac disease, etc.), possibly predisposing to the progression toward an overt hypothyroidism. In children with SH but with no goiter, negative antithyroid antibodies, and TSH <10 mIU/l, replacement therapy is not justified, both because of the low risk to develop an overt hypothyroidism and because they could simply be euthyroid outliers, representing 2.5 % of normal individuals whose TSH values are above 97.5th percentile of euthyroid distribution. Indeed, a slightly elevated TSH may just be the sign of a physiological adaptation in thyroid-healthy children from iodine-replete areas, whose smaller thyroid volume induced by an improved iodine status does presumably require a higher TSH to maintain an adequate thyroid hormone production [56].
References
1.
3.
Canaris GJ, Manowitz NR, Mayor G, Ridgway EC (2000) The Colorado thyroid disease prevalence study. Arch Intern Med 160:526–534PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree