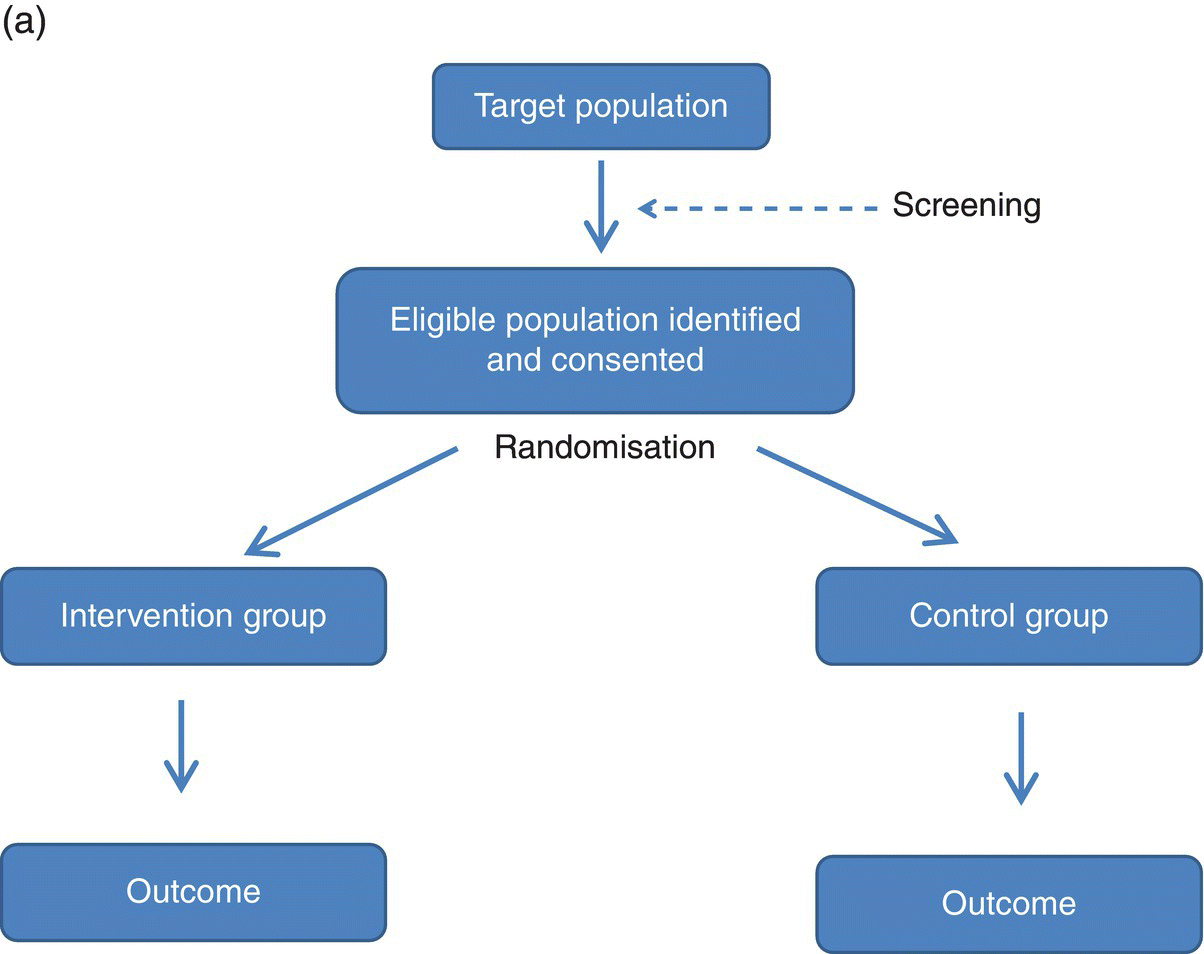
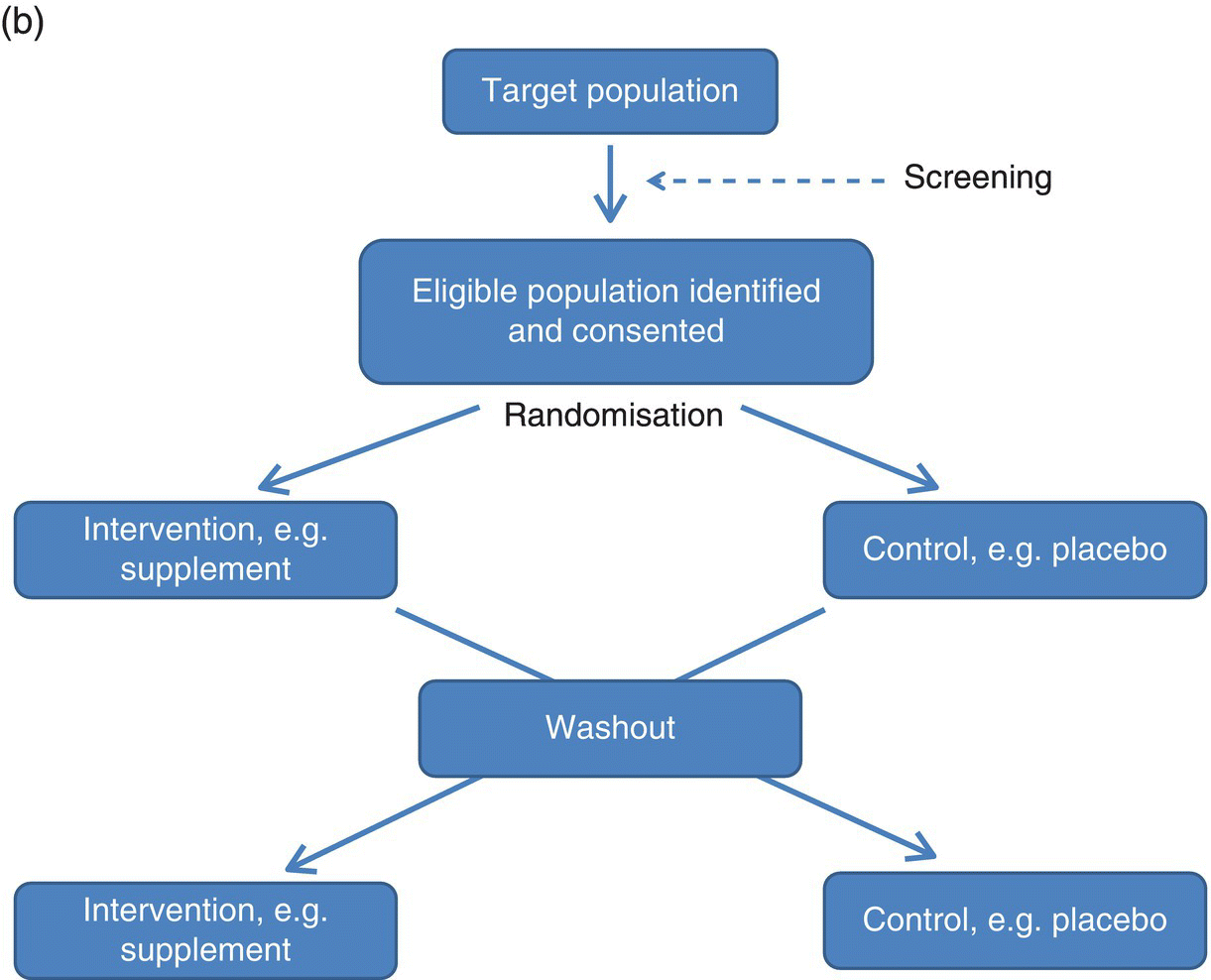
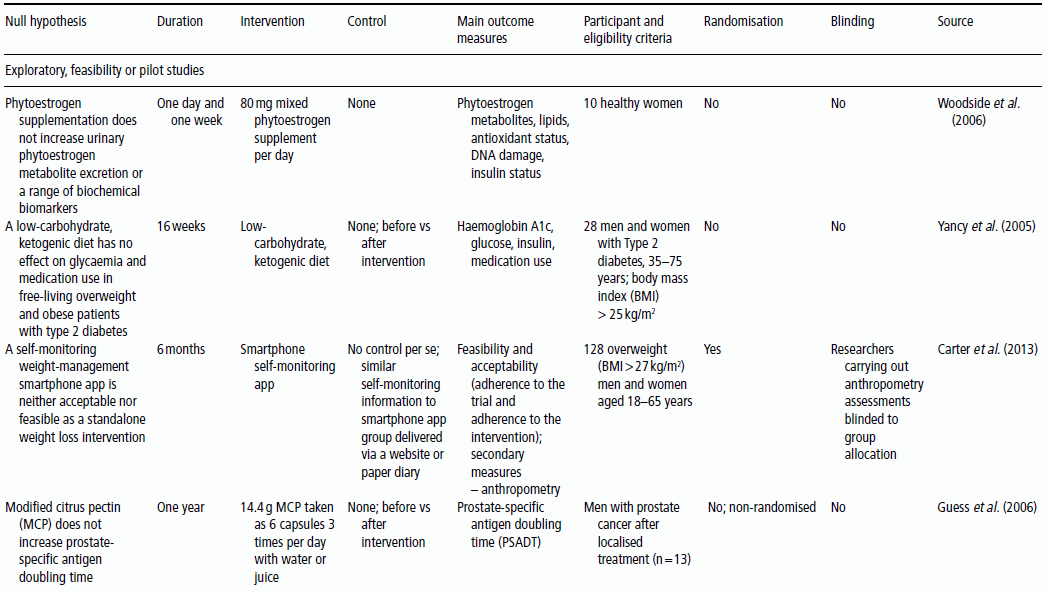
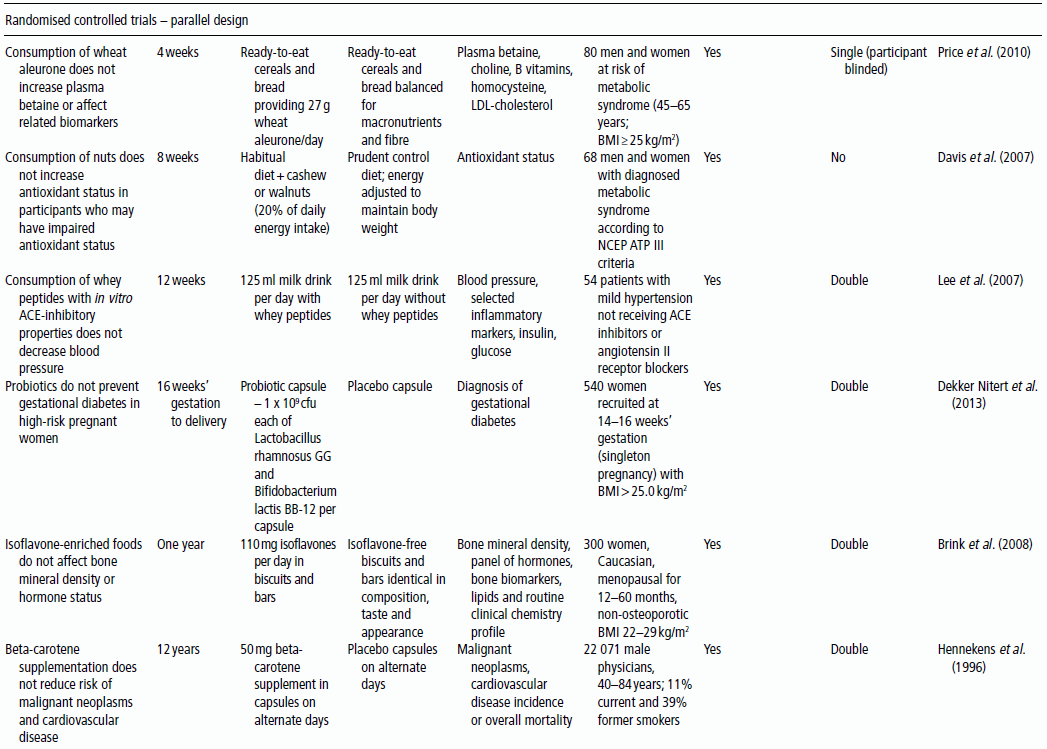
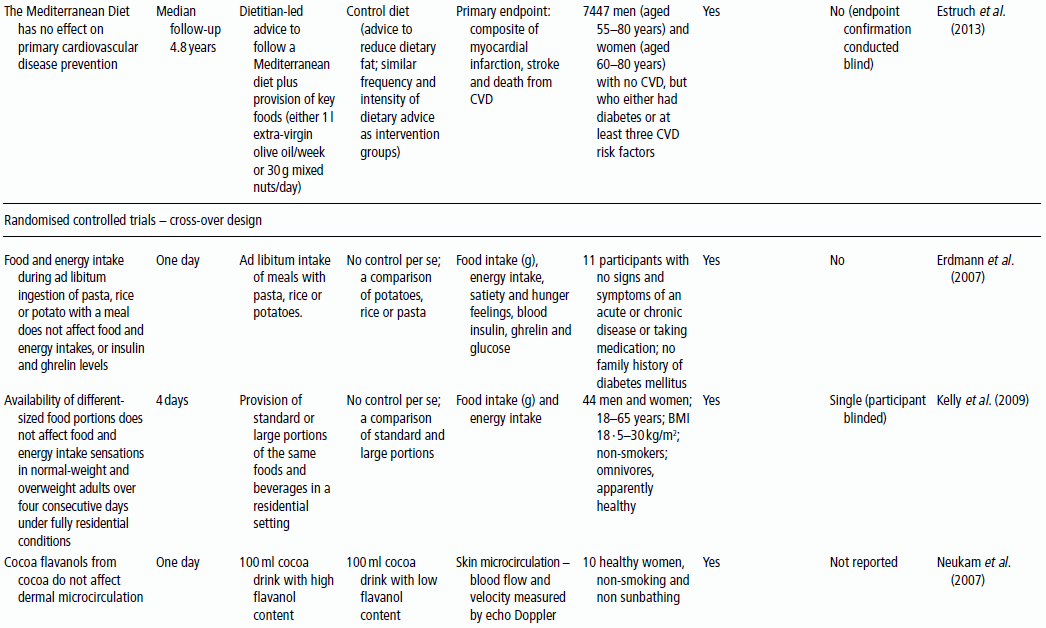
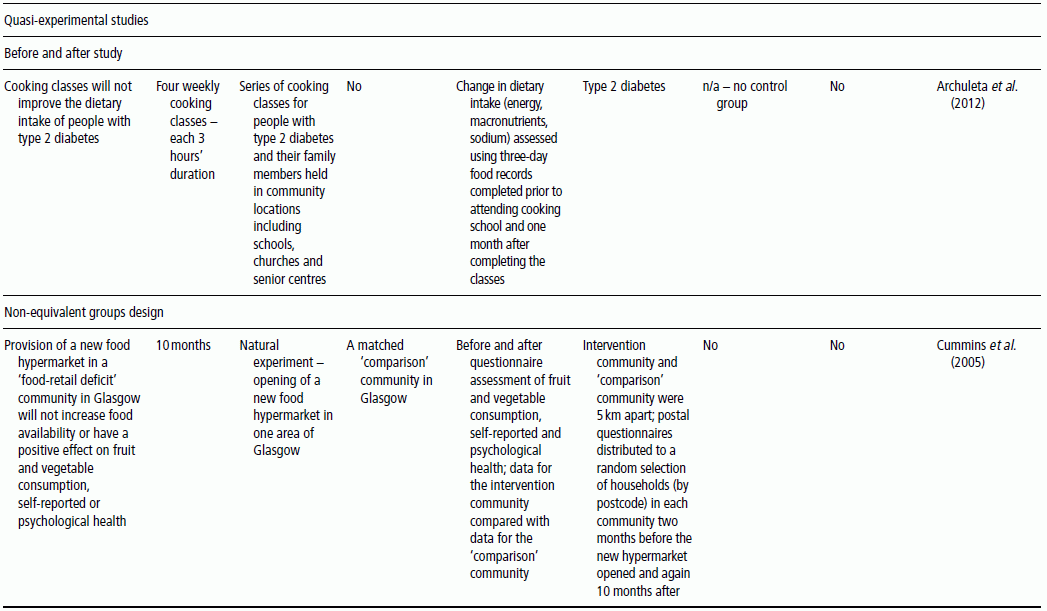
3 Jayne V Woodside,1 Robert W Welch,2 Chris C Patterson1 and Michelle C McKinley1 1 Centre for Public Health, Queen’s University Belfast 2 Northern Ireland Centre for Food and Health, School of Biomedical Sciences, University of Ulster There is substantial evidence linking dietary factors to the primary and secondary prevention of major chronic diseases such as heart disease, diabetes and certain cancers, as well as the improvement of physiological function and the maintenance of adequate nutritional status. Although observational studies (see Chapter 2) can demonstrate an association between a particular nutrient, food or diet and a functional or disease-related endpoint, causality cannot be demonstrated using such study designs. To demonstrate cause and effect requires an intervention study in which consumption of a nutrient, food or diet is altered in a controlled way and the effect on selected outcomes is measured. Intervention studies are higher up the hierarchy of scientific evidence than observational studies, although a combination of different study designs is usually utilised to develop a comprehensive evidence base for a link between consumption of a particular food or nutrient and a health-related outcome. Observational studies often generate hypotheses, which can be tested more rigorously in an intervention study. This chapter will examine the different types of intervention study and then outline some of the key factors to consider when planning such studies. It includes intervention study design when the focus of interest is a particular nutrient, whole food, food group or whole diet, and also discusses nutrient supplementation studies. Intervention studies should be hypothesis driven and have a strong evidence basis. Intervention study designs can range from a short-term study, where the immediate effect of the intervention (consumed once) is measured over minutes to hours (for example, postprandial, or post-meal, studies), through to long-term studies that evaluate the effects of the intervention over a period of weeks, months or years. The study setting can also vary, from those in free-living populations through to studies conducted entirely in purpose-built research facilities such as metabolic suites, or within clinical facilities such as metabolic wards. The main study designs are outlined in this section. Different definitions exist for a pilot study (sometimes the terms ‘feasibility study’ or ‘exploratory study’ are also used interchangeably), but they are generally regarded as studies that are implemented on a small scale in order to test whether all the study processes operate as anticipated before undertaking a full-scale trial. There are many different reasons for performing pilot studies: for example, they are often undertaken in order to assess how realistic it would be to conduct a full-scale trial, to test recruitment procedures in a defined population, to develop and test research instruments, to develop and test a novel intervention (e.g. to evaluate food matrix issues or to ascertain dose or amount to be consumed), to identify logistical challenges in implementing a full-scale trial and to convince funding bodies that such a trial is worth funding. These studies can also provide data on the distribution/variability and timescale of outcome responses, which can be used for power calculations in subsequent definitive studies. Pilot studies vary in design and may test all, or only some, aspects of a full-scale study. They may be single-arm (before and after) studies with no control group, and these can be a cost- and time-effective way of assessing potential effects, but only as a forerunner to controlled studies. Pilot studies add to the totality of evidence, but on their own cannot determine the effect of intervention. In general, data from pilot studies should be reported in descriptive terms and caution should be exercised when interpreting any statistical tests of significance, which will typically lack power. Publishing pilot data provides important insights and information that can be used by other researchers and represents an important element of good study design, particularly for complex interventions. Whole-diet or broader lifestyle interventions, including diet, may be considered ‘complex interventions’, and should be developed according to the UK Medical Research Council’s guidelines on developing and evaluating complex interventions. Development of interventions according to these guidelines will involve the use of qualitative research methods (see Chapter 10). Once these early studies have been completed, studies with greater rigour, in which participants are randomised to study groups, will test the hypothesis that the nutrient, food or diet will alter the selected outcome measures. Usually a series of studies will be conducted, with later studies extending the work as the evidence accrues. Examples include increasing the range of populations studied, using new and/or longer-term outcome measures, assessing the minimum effective amount (or ‘dose’) to be consumed, and evaluating different forms of presentation or delivery of the nutrient or food. In any controlled study, in addition to measuring outcomes in participants receiving the active nutrient, food or dietary intervention, the same outcome measurements will be collected in a control group. The inclusion of a control group, which may receive either a placebo or no intervention, allows control outcomes to be compared with intervention outcomes and therefore increases confidence that changes observed during the study are directly attributable to the intervention. Without a control group, it is inappropriate to make cause-and-effect statements about an intervention, as other factors may be responsible for the effects observed. For example, if a study is conducted over several months without a control group, it is possible that any changes observed are attributable to normal seasonal changes rather than the intervention itself. As well as allowing seasonal variations to be taken into account, having a control group also means that the ‘placebo effect’ can be assessed. In some cases, just taking a supplement or eating in a different way is enough to make an individual feel ‘better’ to some extent, and this is particularly relevant when dealing with more subjective outcomes such as quality-of-life scales. Two basic randomised controlled trial (RCT) study designs are encountered: parallel group studies and cross-over studies. The key features of these study designs are illustrated in Figure 3.1. In parallel group studies, each participant receives only one of the nutrition interventions (e.g. product A or B, or low intake or high intake) under study. Comparisons between groups must therefore be made on a between-participant basis. However, in some studies it may be feasible to use a different design in which participants receive more than one intervention. In cross-over studies, participants receive all interventions under comparison and the design specifies the order of interventions. This has the advantage that comparisons between interventions can be made on a within-participant basis, with a consequent improvement in the precision of comparisons and therefore in the power of the study, and a reduction in the required sample size. In such designs, participants act as their own controls. In a cross-over design for two interventions, the participants are allocated to two groups that receive interventions in a different order. Assessments are performed at the end of each intervention period, although in some cross-over studies baseline measurements may also be taken at the start of each intervention period. Depending on the intervention and outcome measure, a washout period may be required between intervention periods to avoid contamination or carry-over effects; that is, the effect of an intervention given in one treatment period extending into the following treatment period(s). A run-in period may also be desirable in advance so as to minimise order effects. During this period participants may be asked to avoid certain foods. A Latin square design may be used, where appropriate, to extend cross-over studies to more than two interventions. However, since participants receive all interventions, increasing the number of interventions will extend the study duration and so may add to the participant drop-out rate. Figure 3.1 Study design for parallel and cross-over RCTs. Acute studies can follow either of these general designs, but durations will be markedly shorter. (a) Parallel group randomised controlled trial flowchart; (b) Cross-over randomised controlled trial flowchart. For studies that require longer-term interventions, parallel studies are usually preferred, because of their shorter overall time frame. Furthermore, parallel studies are essential where a washout period may be ineffective at returning outcome measures to baseline, for example in certain tests of cognitive function. Parallel studies are also required where intentionally returning to baseline may be unethical, for example if body weight or bone mineral density is the outcome measures. Parallel studies are least suited to outcomes that show large inter-participant variation. Cross-over studies are favoured where participant availability may be restricted and in very short-term studies, for example postprandial studies to evaluate glycaemic responses or effects on satiety and short-term energy intakes. However, they are adversely affected by dropouts and necessitate a more complex analysis methodology. The choice of study design will depend on these considerations, but also on the time frame, the availability of other resources such as cost, the level of financial support and research staff time available, and the potential roles of confounding factors, such as seasonal variations. Using cost as an example to be considered, the sample size for cross-over studies will be smaller, and the time frame for recruitment therefore shortened, but the overall time frame will be longer than for a parallel trial, as participants need to complete both intervention and control arms with an appropriate washout period. The effect of these differences on cost would have to be estimated for each individual study. Other less commonly used types of RCT include the factorial design (in which all possible combinations of two or more interventions are tested, therefore permitting the evaluation of intervention interactions) and the cluster randomised design (in which the unit of randomisation is not the individual but a cluster of individuals defined, for example, by family, school class or primary care group). Further guidance on these designs is available in statistical texts on clinical trials. Like RCTs, quasi-experimental studies are designed to estimate the impact of an intervention on a group of participants. Although they can be similar to RCTs in design, they lack one or more key features of a true experiment; most commonly the element of random assignment to the intervention or control group is absent and sometimes the control group is lacking altogether. Quasi-experimental studies are often used in public health, for example community food-based interventions. They are easier, quicker and cheaper to implement than RCTs and so require less forward planning and a shorter lead-in time. In some situations quasi-experimental studies are the only viable option, as it may not be ethical to have a control group that does not receive any intervention, for example in the provision of vitamins to infants. Some public health interventions, by necessity or for practical reasons, need to be rolled out quickly and on a wide scale, which excludes the incorporation of a control group. Quasi-experimental studies can provide valuable information about the potential usefulness of an intervention, but their internal validity (i.e. their ability to establish causality) will be compromised compared to the RCT design and this limitation must be appreciated when interpreting their results. There are many different variations of quasi-experimental studies, but two frequently encountered designs are: Food fortification is defined by the World Health Organization (WHO) as ‘the practice of deliberately increasing the content of an essential micronutrient, i.e. vitamins and minerals (including trace elements) in a food, irrespective of whether the nutrients were originally in the food before processing or not, so as to improve the nutritional quality of the food supply and to provide a public health benefit with minimal risk to health’. Food manufacturers can fortify foods on a voluntary basis, in line with government legislation, meaning that the individual can make a choice about whether to purchase such foods or not. An example of this would be fortification of ready-to-eat cereals. In contrast, population-based food-fortification programmes are sometimes implemented as part of public health policy to correct dietary deficiencies (e.g. iodised salt to prevent iodine deficiency) or enhance the status of a micronutrient to a level that will prevent specific undesirable health outcomes (e.g. fortification of flour with folic acid to prevent neural tube defects). Population-based fortification programmes require careful planning and consideration of a wide range of background scientific data before commencement, including the following: Furthermore, careful monitoring for undesirable consequences (e.g. over-exposure in certain population subgroups resulting in toxic side effects) as well as desirable effects (improved population micronutrient status, reducing the incidence of the targeted adverse health outcome) of the fortification programme in the short, medium and long term is paramount and should be carefully planned before programme implementation. The major factors involved in the planning, conducting and reporting of intervention studies are identified in Table 3.1, which uses a similar structure to that in the Consolidated Standards of Reporting Trials (CONSORT) checklist for clinical trials. An expanded discussion of these factors appears in this section. These factors will be most relevant for RCTs, as described above, but some factors will also apply to intervention studies in general. Table 3.1 Factors to consider and recommended standards for human intervention trials evaluating health benefits of nutrients, foods and diets. Modified from Welch et al. (2011) and Woodside et al. (2013). Table 3.2 gives some examples, from peer-reviewed journals, of the different study designs described in Section 3.2. Some published studies, particularly earlier ones, do not give clearly stated null hypotheses based on a single primary outcome measure. In these cases, the null hypotheses in Table 3.2 have been inferred from the hypotheses, aims or objectives given in the paper. The distinction between null hypotheses and alternate hypotheses is outlined later in this section. Achieving a study design that fully satisfies all the considerations described here may be constrained in practice by a number of factors, which include practical and logistical issues and the availability of resources, eligible participants and appropriate outcome measures. Thus, the purpose of Table 3.2 is to illustrate the range of types of study design that have been used, rather than to provide examples that may be considered to satisfy fully all the considerations described. Table 3.2 Examples of the different study designs that have been used in human nutrition intervention studies published in peer-reviewed journals. The primary hypothesis, which is tested statistically, should be framed as a null hypothesis, which states that there is no difference between the tested intervention and the control (see Table 3.2). If the statistical test rejects the null hypothesis, then the alternative hypothesis is accepted, indicating that there is a difference between the two interventions. The primary hypothesis to be tested directly influences all aspects of the study, including the study design and duration, the eligibility criteria, the amount of food or nutrient that will be provided and the nature of the control group. The hypothesis should be based on a thorough review of the available evidence. This review should not only encompass other intervention studies, but also consider epidemiological, animal and in vitro studies. Where possible, all available evidence should be reviewed systematically and an assessment of safety and potential risks should be carried out. The primary outcome measure should be clearly defined and must relate to the primary hypothesis.
Study Design: Intervention Studies
3.1 Introduction
3.2 Intervention study types
Pilot studies
Randomised controlled trials: Parallel and cross-over
Quasi-experimental studies
Population-based fortification studies
3.3 Considerations when planning intervention studies
Phase
Factor
Recommended standard
Design
Hypothesis
Clear hypothesis
Study design
Duration
Appropriate design, randomised where possible
Appropriate to design, intervention and outcome measures
Intervention
Amount
Test and control interventions suitably matched
Appropriate to outcome measures and to practical usage
Outcome assessment
Define primary outcome and method of measurement
Define all secondary outcomes and methods of measurement
Eligibility criteria
Define all eligibility criteria
Statistical considerations
Randomisation
Blinding
Size of study
Use randomised design; ensure appropriate allocation, sequence generation and concealment
Ensure double blinding if feasible, single blinding if not
Conduct power calculation based on primary outcome measure
Conduct
Study protocol
Ethical approval and trial registration
Obtain approval, register trial, comply with Declaration of Helsinki
Recruitment
Define recruitment strategy and process, including settings and dates
Data collection
Define relevant measures, select suitable methods for assessment, collection and analysis
– Demographics, lifestyle, background health status and diet, and diet changes
– Adverse events and unintended effects
Use suitable methods to record and respond appropriately
Compliance
Define acceptable level, strive to maximise, assess
Analysis and
interpretation
Statistical analysis
Devise appropriate analysis methods, based on study design and outcome measures
Discussion and interpretation
Consider study limitations and generalisability of findings
Conclusions
Relate directly to hypothesis, study design, intervention and participants
Hypothesis
Duration
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree