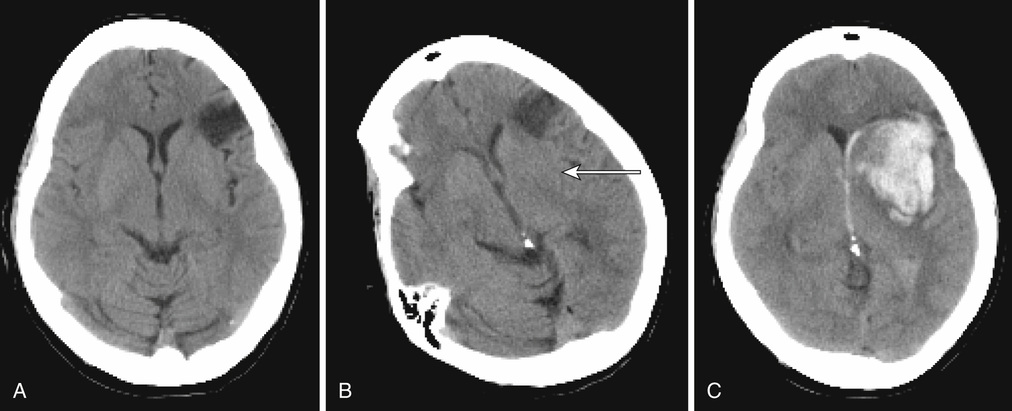
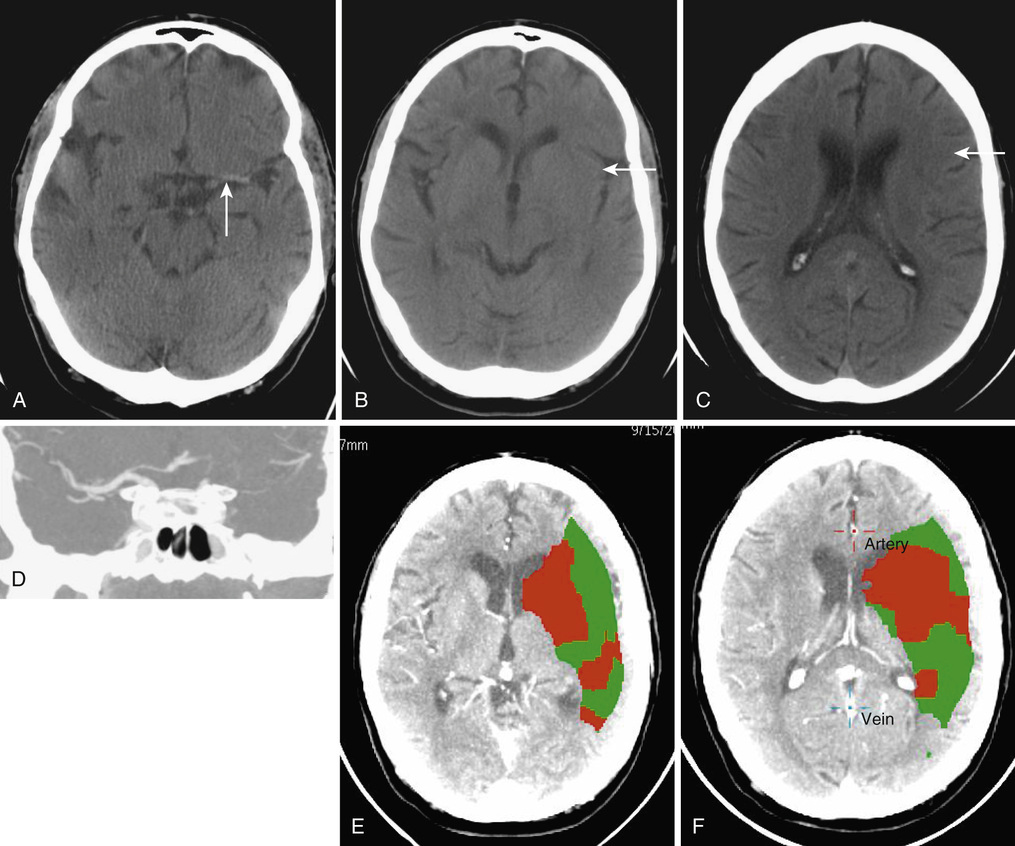
Christopher Moran, Thanh G. Phan, Velandai K. Srikanth Stroke and transient ischemic attacks (TIAs) are the most common clinical manifestations of disease of cerebral blood vessels. Other manifestations of cerebrovascular disease are subclinical and include cerebral white matter lesions, “silent” brain infarcts, and cerebral microbleeds. This chapter focuses mainly on stroke and TIA, with less emphasis on subclinical cerebrovascular disease. In terms of therapy, the chapter does not deal with primary prevention but, rather, with acute treatment, recovery, and secondary prevention. Stroke and TIAs are the leading causes of acute neurologic admissions to hospitals throughout the world and tend to predominantly affect older people. Stroke is the second leading single cause of death worldwide.1 Approximately one third of stroke patients die within the first 6 months, and approximately 60% die within 5 years after stroke.2 Stroke ranks as the sixth most important cause of disability among survivors.3 Increasingly, in the developed world, patients admitted with stroke tend to be frail and have multiple comorbidities. The impact of a stroke on frail older people can be particularly devastating, often leading to a move from their home environment to residential care facilities. It is important to adopt a cohesive and multidisciplinary approach to minimize long-term stroke-related disability and enhance quality of life for the affected person. In the past decade significant improvements in stroke care, based on clinical trial evidence, have been made, and these improvements have resulted in measurable reductions in mortality and disability. The American Heart Association recently defined ischemic stroke (or central nervous system infarction) as “brain, spinal cord, or retinal cell death attributable to ischemia, based on either pathologic, imaging, or other objective evidence of focal ischemic injury in a defined vascular distribution, or clinical evidence of focal ischemic injury based on symptoms persisting ≥24 hours or until death, and other etiologies excluded.”4 Intracerebral hemorrhage is the term applied to sudden focal neurologic symptoms and brain imaging evidence of brain parenchymal hemorrhage. TIAs refer to transient sudden focal neurologic symptoms lasting less than 24 hours and being of presumed vascular origin but without demonstrable infarction or hemorrhage on brain imaging. The type of brain imaging used can make a major difference as to whether a person is diagnosed as having a TIA or stroke. Computed tomography (CT) scans, although sensitive to intracerebral hemorrhage, are relatively insensitive to the presence of early or small infarctions. The use of acute diffusion-weighted magnetic resonance imaging (DWI-MRI) allows the detection of small infarcts in patients who may otherwise be labeled as having a TIA. Nonspecific symptoms such as faintness, loss of consciousness, dizziness, confusion, or falls are highly unlikely to be due to a TIA or stroke, unless they are accompanied by focal neurologic symptoms.5 Acute delirium, a common syndrome affecting older people, is unlikely to last only a few hours and is almost always not secondary to a TIA, although it can be an uncommon presentation of acute stroke.6 Subclinical cerebrovascular lesions are abnormalities detected on MRI brain scans of older people in the absence of a history of acute stroke. They include silent brain infarcts, cerebral white matter lesions, and cerebral microbleeds.7 Silent brain infarcts are usually small subcortical infarcts seen in approximately 10% of the general population older than 65 years, which occur more frequently with increasing age and in the presence of traditional vascular risk factors such as hypertension, smoking, hypercholesterolemia, and diabetes mellitus.7 White matter lesions are visible as hyperintense (bright) signals seen on fluid-attenuated inversion recovery (FLAIR) sequences of MRI scans almost ubiquitously in people aged older than 65 years (their severity increasing with age) and in those with a history of hypertension.7 Cerebral microbleeds are small hypointense (dark) lesions seen on susceptibility weighted imaging MRI sequences and represent hemosiderin deposits adjacent to small vessels. Hypertension, low cholesterol, and the apolipoprotein epsilon 4 (ApoE4) allele are associated with the presence of cerebral microbleeds.8 All three manifestations of subclinical cerebrovascular disease commonly coexist in severe forms in frail older people, can lead to insidious cognitive and motor decline, and increase the risk of both ischemic and hemorrhagic stroke.7 Strokes are either ischemic (80%) or hemorrhagic, each having different pathophysiologic mechanisms and treatments. The mechanisms of arterial occlusion are predominantly those of artery-to-artery embolism and cardioembolism rather than in situ vessel thrombosis. In the absence of arterial venous malformation, aneurysm and cavernous angioma, intracerebral hemorrhage occur in approximately 15% of all cases of stroke, and are either due to hypertensive small vessel disease or amyloid angiopathy.9 Distinguishing ischemic and hemorrhagic stroke is important as their treatments are quite different (thrombolysis and antiplatelet/anticoagulant treatments are used for the former). Some infarcts have hemorrhagic components and may be mistaken for primary intracerebral hemorrhage (Figure 61-1). Separation of these two types of stroke requires careful consideration of the clinical features and their imaging findings.10 The most commonly used classification for ischemic stroke in observational epidemiology is the Oxfordshire Community Stroke Project (OCSP). This classification is based on clinical features and not advanced imaging findings, and hence it is not particularly useful in correctly identifying stroke mechanisms. In clinical trials, the most commonly used criteria for classification are the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria.11 This is a classification of subtypes using a combination of clinical features and results of ancillary diagnostic studies. “Possible” and “probable” diagnoses can be made based on the physician’s certainty of diagnosis based on all available clinical information. The TOAST classification denotes five categories of ischemic stroke: (1) large-artery atherosclerosis, (2) cardioembolism, (3) small vessel occlusion, (4) stroke of other determined cause, and (5) stroke of undetermined cause.11 A feature of this classification is that stroke is attributed to the offending carotid artery if the level of stenosis of that artery is greater than 50%. However, patients can have thromboembolic disease from carotid artery even when the level of stenosis is less than 50%. The degree of stenosis is important when deciding on whether carotid endarterectomy is required, rather than excluding large artery atherosclerosis as a mechanism. The clinical features of TIA and stroke are the results of ischemia affecting eloquent brain areas. The classical patterns of stroke presentations are dealt with later in this chapter but cannot be exhaustively covered in this chapter alone. (For a detailed examination of this topic, see Stroke Syndromes, edited by Bogousslavsky and Caplan.12) However, it must be borne in mind that very old patients (>80 years of age) can have atypical presenting symptoms13 (e.g., falls or reduced mobility) and often have prestroke frailty, and a reasonable index of suspicion for stroke must be maintained for such people. Motor weakness is the most common presenting feature in stroke, affecting about 80% of patients. The pattern of weakness is a clue to the location of the stroke lesion. Unilateral face, arm, and leg weakness often indicates involvement of the middle cerebral artery (MCA) territory, whereas bilateral weakness may indicate posterior circulation involvement. Pure unilateral motor weakness without cortical signs suggests involvement of the subcortical motor tracts (a “lacunar” syndrome). The presence of ideomotor dyspraxia (a disorder of higher cortical disorder of motor initiation) can sometimes mimic motor weakness. Weakness of the articulatory and swallowing muscles can lead to symptoms of dysarthria and dysphagia, respectively, and can occur from strokes affecting both anterior and posterior circulations. Over 60% of stroke patients admitted to the hospital suffer some form of tactile sensory impairment, and smaller proportions either suffer loss of proprioception or have cortical sensory impairment.14 Sensory abnormalities may be associated with delayed but debilitating poststroke pain syndromes.15 Higher cortical deficits that have the most important adverse impact on patients are dysphasia (usually dominant hemisphere stroke) and hemineglect. Broca aphasia (also termed expressive aphasia or motor aphasia) is most commonly caused by strokes involving the left frontal opercular and central cortex, with or without involvement of the subcortical striatocapsular region. It is characterized by effortful speech, word-finding difficulty, phonemic errors, and agrammatism, but comprehension is relatively preserved. Sensory aphasia with relatively fluent speech but poor language comprehension is usually associated with strokes involving the superior temporal lobe and includes Wernicke aphasia and conduction aphasia, among others. Global aphasia refers to severe impairment of motor speech, and comprehension and is usually a consequence of a major left MCA stroke. Hemineglect is characterized by a reduction in attention to stimuli and events on one side of the body and can occur with either right or left hemisphere stroke.16 Hemineglect may affect visual, auditory, and somatosensory perceptual systems and is associated with poor outcome.17 Visual symptoms may arise from lesions affecting the visual pathway anywhere from the retinal to the occipital cortex. Retinal or ophthalmic artery occlusion occurs as a result of embolism from the carotid system and can lead to monocular blindness. Visual field defects are common, leading to either hemianopia or quadrantanopia, depending on the site of the lesion and the extent of damage to the optic radiation. Ocular movement abnormalities are commonly seen in stroke affecting the brainstem, but also less commonly seen with cerebellar and cerebral lesions. Diplopia is usually associated with eye movement abnormality and can be quite disabling. Detection and characterization of visual deficits in stroke patients are of extreme importance, given their potential impact on daily life and complex activities such as driving. Vertigo or a disordered perception of motion of either the patient or the environment can be caused by strokes involving the vertebrobasilar circulation and is often accompanied by nystagmus. Ataxia of the trunk or limbs may be caused by strokes affecting the cerebellum and adjacent brainstem. Several auditory symptoms may also be associated with brainstem strokes including sudden hearing loss, hyperacusis, tinnitus, and auditory hallucinations. Stroke is very commonly associated with neurocognitive syndromes at acute presentation as well as in the medium to long term.18 Close to 50% of survivors have some form of cognitive impairment at 3 months after stroke19; this can occur as a result of the effects of the stroke itself, or it may be a sign of worsening or unmasking of preexisting cognitive decline. Stroke is strongly associated with a twofold increase in the risk of dementia after stroke with the presence of prestroke cognitive decline explaining a large proportion of these cases.20 Up to 30% of patients may also suffer from depressed mood in the medium to long term after stroke.21 Urinary and fecal incontinence are common and disabling effects of stroke. The prevalence of urinary incontinence among survivors of stroke ranges from 36% to 83% within the first year.22 Incontinence may be a direct consequence of loss of neurogenic control or due to functional incapacitation secondary to immobility or cognitive loss, and is a marker for increased mortality after stroke and overall poor outcome among survivors. Features compatible with anterior circulation TIA commonly include unilateral motor, sensory or sensorimotor impairment, dysphasia, and amaurosis fugax. The diagnosis of amaurosis fugax is based on the patient’s report of a transient unilateral visual loss, described on closer questioning as “a curtain coming down” over the affected eye with inability to see through this curtain. Features of posterior circulation TIA include vertigo and/or diplopia, or loss of balance, or unilateral weakness. Computed tomography (CT) should be performed to exclude intracranial hemorrhage. Hemorrhage in the basal ganglia and pons suggests that hypertension may be the likely cause, whereas hemorrhage in cortical (lobar) locations suggests the possibility of amyloid angiopathy as the primary causal mechanism. Signal change on DWI-MRI reflecting altered water diffusion can reveal bright signal abnormalities within minutes of ischemia in the majority of patients with ischemic stroke.25 The signal change on DWI becomes less bright after 10 days.26 Hemorrhage, on the other hand, contains paramagnetic material and has a dark signal on T2-weighted images. The evolution of magnetic resonance (MR) signal changes in intracerebral hemorrhage is complex and the reader is referred to the description by Atlas and Thulborn.27 Stroke pathophysiology can now be inferred from dynamic scanning by tracking a bolus of intravenous contrast with sequential acquisition of CT or MR images. These CT or MR perfusion images enable analysis of cerebral perfusion deficit. For MR images, the salvageable tissue is represented by the difference between the poorly perfused region and the region of restricted diffusion (infarct core).28 For CT perfusion images (Figure 61-2), the infarct core is represented by the most poorly perfused region on CT perfusion images (either cerebral blood volume or relative cerebral blood flow images). The abnormally perfused region is defined by the mean transit time or cerebral blood flow maps. These dynamic scanning methods are used to guide thrombolysis therapy. Extracranial ultrasound can provide evidence of carotid artery and vertebral artery disease, but it has certain limitations. Ultrasound is operator dependent, and assignment of degree of carotid artery stenosis is also dependent on blood flow velocity in that artery. Consequently, a critically narrow artery on one side can lead to compensatory elevation of velocity in the contralateral artery leading to erroneous misclassification of the contralateral artery as critically stenosed. Ultrasound assignment of carotid artery stenosis as moderate (50% to 70% stenosis) can either mean that the artery is between 50% and 70% or greater than 70% stenosed, and a near occlusion can mean near occlusion, complete occlusion, or critical stenosis. A rule of thumb is that when the ultrasound suggests more than 50% stenosis and the patient is fit for carotid endarterectomy, a second test such as CT angiography (CTA) or contrast-enhanced MR angiography (MRA) may be necessary to clarify the exact degree and nature of the stenosis. CTA is performed by rapid injection of contrast bolus and acquiring the images during the arterial phase of contrast arrival in the brain and thus provides coverage from the aortic arch to the circle of Willis. MRA techniques are either “bright blood” or “black blood” techniques depending on the signal intensity of blood. Contrast-enhanced MRA is performed by fast injection of an intravascular contrast agent (gadolinium) and acquiring images during the arterial phase of contrast arrival. CTA and MRA have largely replaced digital subtraction angiography for investigation of carotid artery disease.29,30 An electrocardiogram (ECG) can facilitate identification of atrial fibrillation (AF), which requires anticoagulation for stroke prevention. Ambulatory cardiac monitoring is a useful tool that can assist in detecting paroxysmal AF, which may not show up on an ECG. However, a single episode of monitoring may be insufficient to detect AF, and recent studies indicate a need for longer and more frequent monitoring, which may become feasible with evolving technology.31,32 Echocardiography can assist in relatively uncommon situations where valvular heart disease or endocarditis is clinically suspected as mechanisms underlying ischemic stroke. However, its routine use is controversial. It is rare to find an abnormality on an ECG that would lead to anticoagulation in patients with normal ECG results and cardiovascular examination. Routine echocardiography may lead to the chance finding of patent foramen ovale, which may further confuse clinical decisions, as the evidence is lacking for endovascular therapy in such situations.33–35 Complex aortic arch atheroma confers a fourfold increased risk of stroke.36 While transesophageal echocardiography is superior to transthoracic echocardiography for the detection of aortic arch atheroma,37 there is no evidence at this time whether warfarin may be more useful than routine antiplatelet therapy in patients with arch atheroma.36 Blood tests may be of some use in assessing stroke risk in the acute setting, but their actual use is relatively limited for this purpose. Serum cholesterol levels dip acutely after stroke and return to “true” values approximately 12 weeks after stroke onset.38 Similarly, acute phase hyperglycemia may occur in stroke patients irrespective of diabetes mellitus, and hence it is advisable to conduct definitive testing for this a few weeks after stroke. Inflammatory markers such as erythrocyte sedimentation rate and high-sensitivity C-reactive protein can be performed to examine the possibility of temporal arteritis or subacute bacterial endocarditis, but more often these markers are useful in determining the course of poststroke infections. Routine testing for antiphospholipid antibody levels is unhelpful because of its high prevalence in people older than 40 years,39 poor specificity for stroke risk,39,40 and lack of utility in determining therapy.41 In older patients, a full blood count can be helpful to exclude uncommon thrombotic disorders such as thrombocythemia or polycythemia rubra vera, but it is unlikely that a routine search for other rare thrombophilic causes of stroke will be useful. The implementation of organized stroke units has been the most important advance in acute stroke management. A collaboration of stroke unit trialists demonstrated that organized stroke units need to treat approximately 25 patients to prevent one from dying or being dependent.42 Outcomes were also better in patients admitted to a discrete ward under the care of a dedicated multidisciplinary team, compared with a roving stroke service visiting patients on general medical wards.43 The consistent characteristics of effective stroke units appear to be (1) a comprehensive approach to medical problems, impairments, and disabilities; (2) active and careful management of physiologic abnormalities; (3) early mobilization; (4) skilled nursing care; (5) early setting of rehabilitation plans; and (6) early assessment and planning of discharge needs with involvement of caregivers.44 Appropriate fluid and nutritional support in the acute phase with either intravenous fluids or nasogastric feeding are also important in those who have significant dysphagia and risk of aspiration. Early nasogastric feeding, within the first week after stroke, has been associated with reduced mortality but with an increase in dependency on others for activities of daily living.45 Therefore, percutaneous endoscopic gastrostomy should be reserved for only those who require long-term care and need assisted feeding because of severely impaired swallowing. The early detection and treatment of pyrexia and infectious complications is a major contributor to the effectiveness of stroke unit care.46 A protocol-driven approach in stroke units can also facilitate the standard use of intermittent pneumatic compression devices to prevent venous thromboembolism, with recent evidence supporting their superiority in ischemic stroke compared with low-molecular-weight heparin or graduated compression stockings.47 There is no evidence of benefit of the use of heparin or its derivatives in the prevention of venous thromboembolism in patients who have acute ischemic stroke.48 Nursing care should incorporate avoidance of pressure areas, urinary catheters, and bed rest because these contribute significantly to the development of complications such as sepsis and deep venous thrombosis. Blood pressure reduction should be generally avoided in the acute phase (except in selected situations such as before thrombolysis or in people with hemorrhagic strokes and mass effect) because of concerns about interfering with cerebral autoregulation; if performed, it should be done cautiously and in well-monitored situations. A very important component of stroke unit care is the conduct of regular (weekly) formal multidisciplinary meetings, which serve as forums for the entire team to discuss various aspects of individual patient care and set early plans for rehabilitation and discharge.44 There is emerging evidence that prestroke frailty is an important determinant of poststroke complications and outcome.49,50 Stroke unit care provides the ideal setting for careful multidisciplinary decision making regarding the use of acute thrombolysis, blood pressure control, medication management, treatment of infection, and early mobilization while taking into account degree of frailty. Three specific interventions have been shown to be effective in randomized trials for the acute treatment of ischemic stroke; these are antiplatelet agents,51 tissue plasminogen activator (tPA), and endovascular therapy.52–54 Neuroprotective agents have largely failed to show benefits in acute stroke therapy. The International Stroke Trial (IST) and Chinese Aspirin Stroke trial (CAST) clearly demonstrated the efficacy of aspirin (160 to 300 mg) as an acute stroke therapy for ischemic stroke.51,55 Of several trials that studied the efficacy of low-molecular-weight heparin in acute ischemic stroke, none showed superiority over aspirin but with increased risk of intracranial hemorrhage.56 The introduction of recombinant tPA has led a paradigm shift in reversing the neurologic deficit caused by acute ischemic stroke.52,53 It is postulated that the mechanism of action of tPA is lysis of the thrombus/embolus leading to recanalization of the arterial lumen and salvage of the ischemic brain tissue. The beneficial effect of tPA was greatest in those treated within 3 hours of stroke onset, may be beneficial up to 6 hours,57 and is now recommended for use within 4.5 hours after onset. The most important adverse effect of tPA is symptomatic intracerebral hemorrhage in about 6% of cases. Symptomatic intracerebral hemorrhage accounts for most of the early excess deaths among those treated with tPA (odds ratio, 3.72; P < .0001), and its risk rises with age, high blood pressure, and very severe neurologic deficits.1 Despite concerns regarding an increased risk of bleeding in older people, those older than 80 years appear to receive similar benefits from tPA as those younger than 80 years.57–59 Research is also under way to identify thrombolytic agents with lower risk of hemorrhage which may be safer to use in older age groups.60 However, there are some important caveats to using thrombolysis in older patients. The presence of a significant preexisting dementia or extreme frailty (particularly in those already living in high-level residential care) may be cause for concern and be relative contraindications to thrombolysis. Older patients with extensive cerebral white matter lesions or microbleeds visualized on brain imaging may be more likely to develop thrombolysis-related intracranial hemorrhage.61 Treatment decisions in such frail older patients must be based on individually estimated risks and benefits. In addition to intravenous thrombolysis, there has been a significant advance with respect to the introduction of endovascular therapy for acute ischemic stroke. In 2015, there were five published randomized controlled trials in people aged 18 to 80 years, showing a large benefit for clot extraction with a stent retriever device in addition to intravenous therapy for proximal arterial occlusion in the anterior circulation compared with intravenous therapy alone.54 Benefits included a greater number of patients discharged with no disability, reduction in disability, and reduced length of stay. The 2015 American Heart Association/American Stroke Association (AHA/ASA) guidelines recommends this therapy as having class 1, level A evidence for the treatment of acute ischemic stroke resulting from occlusion of the MCA.54
Stroke
Clinical Presentation, Management, and Organization of Services
Introduction
Definitions
Stroke and Transient Ischemic Attack
Subclinical Cerebrovascular Lesions
Stroke Types
Ischemic Stroke Subtypes
Clinical Presentation of Stroke and Transient Ischemic Attack
Clinical Features of Stroke
Clinical Features of Transient Ischemic Attack
Investigations for Stroke
Brain Imaging
Diagnosis of Hemorrhage
MRI Findings in Stroke
Evaluating Tissue at Risk
Diagnosis of Stroke Mechanism
Vascular Imaging
Cardiac Investigations
Blood Tests for the Diagnosis of Stroke Risk Factors
Management of Stroke Patients
Organized Stroke Unit Care
Treatment of Acute Ischemic Stroke
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Stroke
61