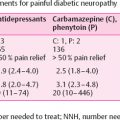
Introduction
Peripheral somatic and autonomic neuropathy are major risk factors predicting future mortality in diabetes . Therefore prevention and treatment of neuropathy are important not only in reducing the patient burden from this common complication but is also vital in reducing overall morbidity and mortality in diabetes. At the present time, there are no medications that have been proven to be effective in randomized, blinded (masked) clinical trials. Numerous clinical trials aimed at a diverse array of biochemical pathways have failed, up to this point, to show efficacy for diabetic polyneuropathy. This failure of clinical trials is likely due to several causes. First, the pathophysiology of diabetic neuropathy is extremely complex, and it is likely that more than one treatment approach will be required to achieve clinical improvement. Second, stabilization or improvement in diabetic neuropathy may take years. A small treatment effect may be missed by trials, which because of research funding limit treatment to 1 year or less. Third, the endpoints and the trial design that have been used may lack adequate sensitivity to detect small changes in the severity of diabetic polyneuropathy. Fourth, and perhaps most importantly, many of the trials of diabetic neuropathy have approached treatment when the patient’s disease has progressed to the point where nerve regeneration and concomitant improvement in symptoms and signs of disease are limited. Treatment of diabetic neuropathy early in the disease before there is significant neurodegeneration is critical if new therapies are to show efficacy in clinical trials and be introduced into practice.
Although there are no specific medications to prevent or treat the pathophysiology of diabetic neuropathy, there are approaches that can slow the progression of neuropathy and may impact mortality from the disease. These approaches are discussed in the following sections and include improved glycemic control for neuropathy associated with type 1 diabetes mellitus (T1DM), lifestyle interventions, nutraceuticals, and medications that require further clinical testing.
Improved glycemic control
Intensive glucose control with the goal of normoglycemia is one of the mainstays of treatment in diabetes . Strict insulin therapy has been shown to be effective in the prevention or delay of progression of diabetic polyneuropathy (DPN) in T1DM patients . However, in type 2 diabetes mellitus (T2DM), the evidence that improved glycemic control can slow progression of neuropathy is limited . Seven of the studies addressing the effect of improved glycemic control examined DPN in T1DM; however, only two reported outcomes related to clinical impairment. Overall, there is a large, significant reduction in neuropathy with tighter glucose control in T1DM. There have been eight randomized clinical trials of patients with T2DM, and the results have been less definitive than those in T1DM. Half of these studies examined the effects of glucose control on clinical impairments from neuropathy. Overall, there is evidence of a modest reduction in neuropathy in patients with T2DM receiving enhanced glucose control, which is in contrast to the substantial effect seen in those with T1DM . Explanations for this difference may be because of the different outcome measures used in the trials, different treatment regimens utilized for glycemic control, the higher incidence of DPN in controls with T2DM, and differences in baseline glucose control among the clinical trials. Importantly, this difference in the impact of glucose control on DPN in T1DM and T2DM highlights the differences in the disease mechanisms and complications between these two diseases.
Somatic neuropathy in type 1 diabetes mellitus
One of the largest trials that established that tight glycemic control can effectively delay the onset or slow the progression of neuropathy in T1DM was the Diabetes Control and Complications Trial (DCCT) . This trial of over 1400 patients with T1DM found that intensive diabetes therapy with either multiple daily insulin injections or continuous subcutaneous insulin infusion reduced the development of clinical neuropathy by 64% (95% CI, 45%–76%) compared to conventional therapy over 5 years. The prevalence of abnormalities on nerve conduction testing was reduced by 44% (CI, 34%–53%) in the intensive treatment group compared to conventional treatment. In terms of neuropathy progression, the DCCT found that intensive glycemic control was effective in preventing decreases in nerve conduction velocities (NCV) observed in participants in the conventional treatment cohort .
The question of whether normoglycemia can prevent the development of neuropathy in T1DM was examined by a prospective observational study of 11 patients with well-controlled T1DM (mean HbA1c<7.0%) compared to 21 patients with poor control (mean HbA1c>7.0%) . The participants were followed from the time of diagnosis (duration of diabetes 2.7+0.3 weeks) for over 24 years. None of the patients with well-controlled diabetes developed clinical polyneuropathy. Compared to participants with poorly controlled T1DM, there was significantly less decline in peripheral and autonomic nerve function as measured by motor and NCV, quantitative sensory testing, and heart rate variability.
At the completion of the study intervention in the DCCT, the participants originally assigned to conventional insulin treatment were encouraged to begin intensive treatment. Despite similar HbA1c levels 13–14 years later, the participants who were originally assigned to the conventional treatment group continued to develop neuropathy at a higher rate than those originally assigned to the intensive insulin therapy . The persistent effect of early intensive insulin therapy is referred to as “metabolic memory,” which has been ascribed to a change in the individual’s epigenetic expression. Support for this concept was provided by evidence of differences in epigenetic DNA methylation during the DCCT that were found to persist at certain loci associated with glycemia during the Epidemiology of Diabetes Interventions and Complications (EDIC) study .
Based upon the beneficial effects of normoglycemia achieved by intensive improvement in glycemic control, several small trials have examined the effects of simultaneous pancreas and kidney transplants on neuropathy in T1DM. Overall, transplantation is associated with both early and maintained small nerve fiber regeneration, followed by improvement in nerve conduction indices. Improvements in corneal confocal microscopy assessments, a measure of small fiber pathology, have been seen within 6 and 12 months of transplantation . A controlled, 3-year-long observational study found that, when compared to the patients with T1DM and severe DPN who did not undergo transplant, those in the transplant group saw improvements in the corneal nerve fiber density and length that were maintained over the course of the study. While the intraepidermal nerve fiber density (IENFD) did not change, the mean dendritic length improved significantly at 12 and 36 months. By 36 months, there were also significant improvements in the neuropathy symptom profile and peroneal NCV. There was no change in quantitative sensory testing or cardiac autonomic function assessments . The long-term effects of simultaneous pancreas and kidney transplantation on patients with T1DM and severe neuropathy at baseline have also been examined. An observational study of 12 participants with T1DM and severe neuropathy prior to transplant found that after 8 years, all the participants had excellent glycemic control (median HgBA1c 5.5%) but there was no improvement seen in IENFD, vibration perception threshold (VPT) or autonomic function testing results. However, a statistically significant improvement in the median motor NCV was seen . The results from this long-term study highlight the fact that advanced DPN is often irreversible and supports the idea that early prevention of neuropathy is paramount.
Somatic neuropathy in type 2 diabetes mellitus
In contrast to T1DM, there is no convincing evidence in T2DM patients that intensive glucose control can prevent or delay the development or progression of DPN. There is evidence to support an important role of glucose control in DPN associated with T2DM, but normoglycemia alone is not sufficient to prevent or reverse DPN. A Cochrane systemic review in 2012 concluded that while enhanced glucose control prevents the development of neuropathy in patients with T1DM, there is a nonsignificant reduction in the incidence of clinical neuropathy in patients with T2DM. However, there is a significant reduction in nerve conduction and vibration threshold abnormalities .
One of the largest trials of the effect of normoglycemia on neuropathy in T2DM is the UK Prospective Diabetes Study (UKPDS) . The UKPDS randomized 3867 patients with newly diagnosed T2DM to either intensive glucose therapy with a sulphonylurea, intensive glucose therapy with insulin, or conventional diet therapy. At the end of 10 years, there was a small difference in HgBA1c between the intensive (HgBA1c 7.0%) and standard (HgBA1c 7.9%) control groups. An aggregate 25% risk reduction ( P =.0099) in microvascular endpoints was found in the intensive blood-glucose groups after 10 years. The main neuropathy outcome measure, vibration detection threshold, had a lower rate of impairment with intensive glucose therapy compared to conventional therapy. However, this difference was only significant after 15 years (relative risk 0.60, 95% CI 0.39–0.94). More importantly, despite the small improvement, there was a higher rate of hypoglycemic complications in the intensive glucose therapy groups, particularly with insulin treatment.
There have been other more recent large studies of the effects of strict glucose control on the development of T2DM complications. The VA Diabetes Trial randomized 1791 military veterans with T2DM to either intensive or standard glucose control. The primary outcome measure was time to a major cardiovascular event. The development of neuropathy was determined based upon self-report. While they did not find any difference in the rate of new microvascular complications, including neuropathy, there was a nonsignificant 5% reduction in the incidence of neuropathy . The Action to Control Cardiovascular Risk in Diabetes trial was also designed to determine if strict glycemic control in T2DM was effective in reducing the rate of cardiovascular events . This trial randomized 10,251 patients to either intensive glucose control (HgBA1c goal<6.0%) or standard care (HgBA1c goal 7.0%–7.9%). The trial was stopped early after 3.5 years due to higher mortality in the intensive therapy group; however, planned follow-up continued for 5 years. The incidence of neuropathy, as measured by a Michigan Neuropathy Screening Instrument (MNSI) score>2.0 was significantly reduced in the intensive glucose control group (Hazard Ratio (HR) 0.92, Confidence Interval (CI) 0.86–0.99, P =.027, Number Needed to Treat=33). Intensive treatment was also associated with significant reductions in the development of loss of ankle jerk and light touch by 10-g monofilament, but the loss of vibratory sensation did not differ.
Additional evidence from smaller trials of patients with T2DM lend support for the role of glucose control in preventing the progression of neuropathy. A Japanese trial enrolled 110 patients with T2DM who were randomly assigned to intense or conventional insulin treatment. Intensive insulin therapy group showed significant improvement in NCV and vibration threshold while those in the conventional treatment group showed deterioration in these measures . More recently, another study assessed patients with uncontrolled T2DM and DPN at baseline and determined the effect of intensive glucose treatment on measures of small fiber neuropathy . Patients were treated with either standard care or intensive HbA1c control, which included diet restriction and hypoglycemic agents, and were then followed for an average of 4 years. The participants who were treated with intensive HbA1c control demonstrated normalization of HbA1c (9.6%–5.9%), which was maintained for 2 years. At the end of the study, there were significant improvements in several measures of nerve conduction studies as well as corneal nerve fiber measures seen in the group treated with extensive HbA1c control, while the vibration detection threshold improved in the standard care group. These improvements were associated with a significant reduction in weight in the extensive HbA1c control group.
Not all studies have shown that aggressive glucose control reduces the development or progression of neuropathy in T2DM. The Veterans Affairs Cooperative Study on Type II Diabetes Mellitus (VA-CSDM) was designed as a feasibility study that compared standard insulin treatment to an intensive therapy group in men with T2DM who required insulin. The enrolled men had poorly controlled T2DM with an average duration of 7.8±4 years and about half had neuropathy at baseline. Intensive insulin therapy did result in an improvement in HgBA1c after 6 months and was 2% lower than that in the standard treatment group. However, there was no difference found in the prevalence of DPN between the intensive versus standard arms (64% intensive vs 69% standard) after 2 years .
Glucose control is probably only part of the optimal treatment approach to DPN, and the type of glucose-lowering agent used to obtain normoglycemia may play a role in preventing the development of DPN in T2DM. The Bypass Angioplasty Revascularization Investigation 2 Diabetes trial enrolled 2159 participants with T2DM and coronary artery disease to examine the effect of insulin-sensitizing treatments (metformin, thiazolidinediones, or both) versus insulin-providing treatments (sulfonylureas/meglitinides, insulin, or both) on cardiovascular outcomes . DPN was a predetermined secondary outcome measure and was defined as an MNSI clinical examination score>2. After adjusting for HbA1c, which was significantly lower in the insulin-sensitizing therapy arm, the cumulative incidence of new onset DPN in those participants without DPN at baseline ( n =1075) over 4 years was significantly reduced in the group of patients treated with insulin-sensitizing therapies compared to those receiving insulin-providing therapies (66% vs 72%, P =.02). There was no difference between the two treatment arms in the prevalence of DPN (51% vs 53%) and there was no difference in the remission rates in those who had established DSP at baseline. There was a significant association between changes in the MNSI questionnaire and changes in body weight, HgBA1c, and serum lipids. The finding that insulin-sensitizing treatments are associated with a reduced development of DPN in T2DM, when compared to insulin-providing treatments, indicates that the effect of glycemic control may be pathway specific.
The methodological weakness in these trials is that most of the trials only enrolled a minority of patients with DPN, only included a few clinical endpoints of DPN, different endpoints were used, only a few included quantitative measures of small fiber pathology, DPN and cardiac autonomic neuropathy (CAN) were secondary and not primary outcomes, and the duration of intervention was relatively short.
Diabetic autonomic neuropathy
Data regarding the effect of strict glucose control on CAN in T1DM and T2DM is similar to that for somatic DPN. In patients with T1DM, the data for diabetic autonomic neuropathy (DAN) from the DCCT and the follow-up EDIC study was more conclusive for CAN than it was for DPN. The prevalence of CAN was low at the start of the DCCT. While the prevalence of CAN almost doubled in the conventional group, it remained stable in the intensive group and the risk reduction in incident CAN with intensive therapy was 45%. At the end of the DCCT, both groups were instructed to adhere to the intensive therapy and were followed in the EDIC study, which had more conclusive data for CAN than it did for DPN. After 13–14 years of observation, the prevalence of CAN increased in both groups but remained significantly lower in the former intensive control group compared to the former conventional group (28.9% vs 35.2%, P =.018). After adjusting for covariates, former participation in the intensive glucose control arm of the DCCT reduced the risk of incident CAN by 31% (OR 0.69, 95% CI 0.51–0.93) . It is possible that the more robust data for the effect of early, strict glycemic control on CAN as opposed to DPN may be attributed to the choice of outcome measures. The effect of metabolic memory may be greater on the small unmyelinated nerve fibers that are measured with the DCCT autonomic measures (R-R variation with paced breathing, Valsalva ratio, and postural blood pressure changes) as opposed to the tests of large fiber function (nerve conduction studies and VPT) that were chosen as measures of DPN.
Similar to DPN, the data for the effect of intensive glycemic control on CAN in T2DM is not as convincing as for T1DM. The lack of sensitive, standardized outcome measures is problematic for both DPN and CAN and there is even less data on outcomes of autonomic function. The results from the United Kingdom Prospective Diabetes Study (UKPDS) and the Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen Detected Diabetes in Primary Care (ADDITION)-Denmark trials found that intensive glucose control in participants with newly diagnosed T2DM had no effect on CAN compared to standard care. However, there were no measures of neuropathy included in the observational follow-up of the UKPDS, and the ADDITION-Denmark study did not include any baseline evaluations for CAN. Similarly, the Veterans Affairs Diabetes Trial reported a nonsignificant increase in CAN in the intensive therapy group and the VA-CSDM found no difference in CAN after 2 years of intensive insulin treatment.
The Steno type 2 study also examined the effects of intensive treatment of T2DM and included CAN as a secondary endpoint . This study utilized an intensive multifactorial treatment, which included not only intensive glycemic control but also treatment of hypertension, dyslipidemia, and microalbuminuria in T2DM. Over approximately 4 years of follow-up, intensive treatment was found to be associated with a lower rate of progression of DAN compared to standard care (OR 0.32, CI 0.12–0.78).
Lifestyle interventions
Traditionally, DPN was considered to be a late complication of diabetes with no effective treatments other than symptomatic neuropathic pain control. However, a growing body of evidence indicates that small fiber neuropathy can occur early in the disease course at the earliest stages of glucose dysregulation. This is an important finding with significant clinical implications because the unmyelinated small nerve fibers that are vulnerable to effects from metabolic derangements are also more responsive to therapies compared to large myelinated fibers . Evidence for an association between prediabetes and small fiber neuropathy comes from studies demonstrating an increased prevalence of impaired glucose tolerance (IGT) in patients with idiopathic DPN and DAN compared to controls . In addition, the prevalence of DPN in individuals with both impaired fasting glucose and IGT (23.9%) has been found to be close to those with known T2DM (22.0%) . There is less data regarding the association between prediabetes and DAN, but the German KORA S4 study found similar prevalence rates of CAN in participants with newly diagnosed T2DM and those with combined impaired fasting glucose and IGT . However, no difference in the prevalence of DPN was found in patients with impaired glycemia compared with matched controls with normoglycemia in a population-based study in Olmsted County, MN, USA . However, this study has several methodological problems: (1) The study used relatively insensitive measures of neuropathy as endpoint measures, for example, composite scores of nerve conduction (which are frequently normal in prediabetes) and quantitative sensation tests, rather than using more sensitive measures of early neuropathy such as the IENFD; (2) rather than using prospective data capture for baseline “glycemic groups,” a reclassification was applied to the existing glycemic data; (3) if the “normative” comparison data is obtained from a population group that is already known to be prone to metabolic syndrome, then the comparator data may be inadequate to detect a difference between groups; (4) the study did actually show decreased heart rate response to deep breathing between the impaired glycemia (nondiabetic) group and the nonimpaired glycemia (healthy subject) group and there was no difference in decreased heart rate response to deep breathing between the impaired glycemia and diabetic groups. Heart rate responses to deep breathing, including the inspiration:expiration ratio, have been shown to be sensitive and reproducible measures of CAN . Another reason for the conflicting data on the relationship between prediabetes and neuropathy is that impaired glycemia alone is not enough to cause neuropathy and there must be another metabolic driver(s) for the onset and progression of neuropathy.
Metabolic syndrome refers to a collection of abnormalities that occur together and include hypertension, hyperlipidemia, central obesity, and insulin resistance. Having metabolic syndrome has been found to increase an individual’s risk for heart disease, stroke, and diabetes. While knowledge of how metabolic syndrome and its individual components effect peripheral nerves is growing, metabolic syndrome has been linked to an increased risk of neuropathy. In patients with T2DM, those with metabolic syndrome were found to be twice as likely as those with T2DM alone to have neuropathy . In nondiabetic patients, metabolic syndrome has been found to be associated with a small fiber neuropathy . As described above, strict glucose control alone is not enough to halt the progression or reverse DPN in T2DM. Components of metabolic syndrome, specifically obesity and dyslipidemia, have been identified as risk factors for DPN independent of glucose control . Dyslipidemia is thought to play a critical role in the development and progression of DPN through associated metabolic, endocrine, and inflammatory effects and are attractive targets for disease-modifying treatment of DPN .
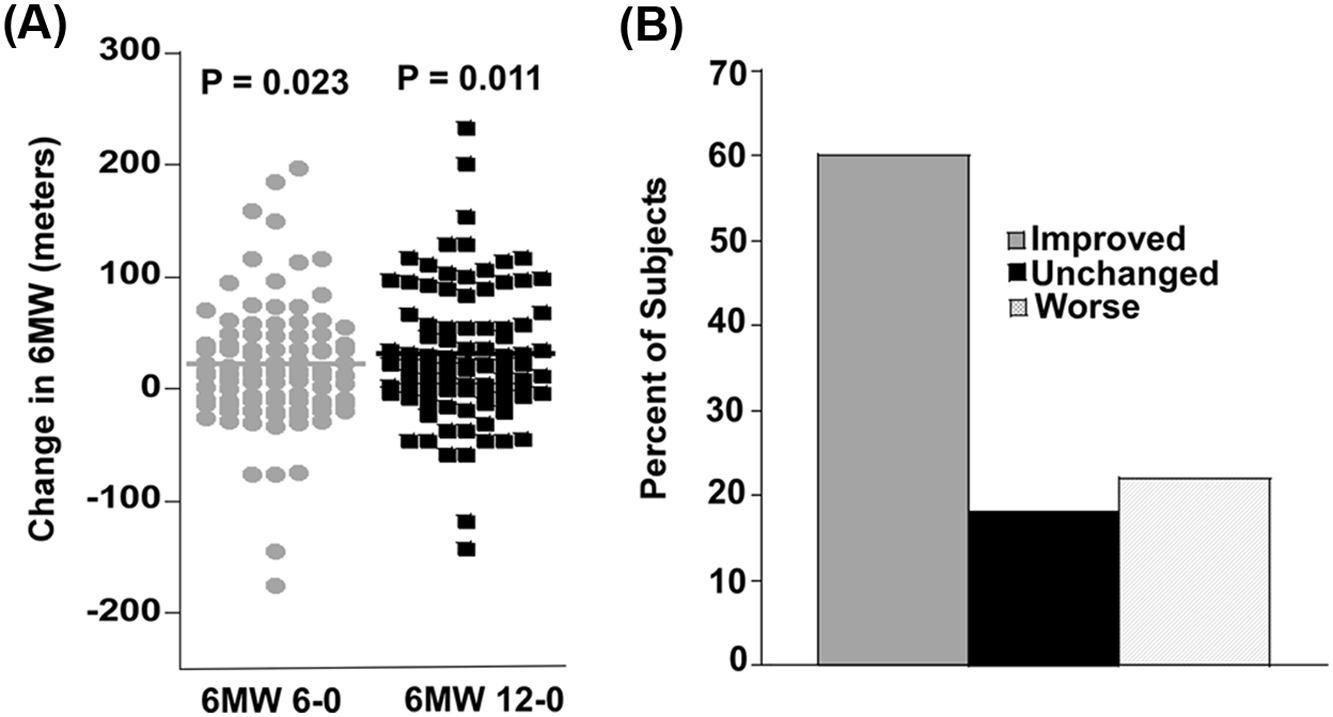
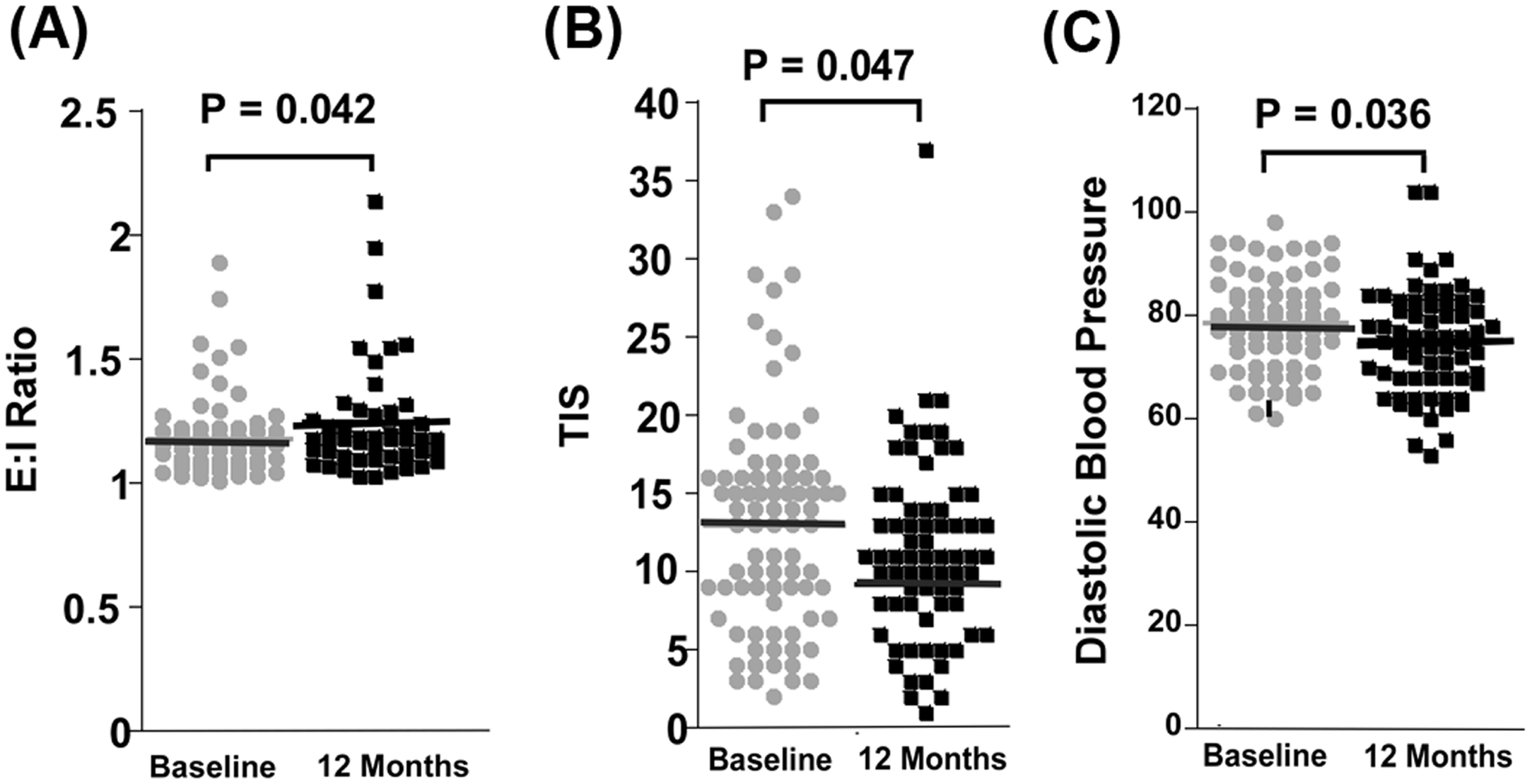
One way to target several of the components of metabolic syndrome as well as many of the metabolic and inflammatory pathways involved in the pathogenesis of DPN is through aerobic exercise. The pathophysiology of DPN involves persistent hyperglycemia as well as multiple biochemical pathways that ultimately result in oxidative stress and inflammation. In contrast to targeted therapies, aerobic exercise has multiple synergistic effects on many of the pathways that are adversely affected by diabetes. Exercise not only improves insulin sensitivity and glucose control, but it increases end-organ perfusion, reduces lipid and protein oxidation, inhibits adipocyte production of free fatty acids and deleterious adipokines, and reduces humeral inflammation . Furthermore, glucose-modifying drug therapy is typically not appropriate in patients with prediabetes due to cost and potential for serious side-effects such as hypoglycemia. A more suitable approach for these patients would be a lifestyle intervention that could arrest the underlying process that leads to neuropathy and its associated functional disability. Exercise has been shown to improve neuropathy function, for example, an improvement in the 6-minute walk test ( Fig. 16.1 ) and neuropathy symptoms and measures of somatic and autonomic neuropathy ( Fig. 16.2 ). In addition, exercise can promote regrowth of cutaneous small diameter nerve fibers in both animal models and human trials, but data from well-designed randomized control trials is needed .
There have been several studies that found that lifestyle interventions are effective in preventing or delaying the onset of T2DM in people with IGT. A 6-year long lifestyle intervention in people from Da Qing, China with IGT found a 43% reduction in the incidence of diabetes in a combined diet and exercise intervention group compared to the control group . The participants were followed for an additional 20 years and the combined lifestyle intervention was associated with a 47% lower incidence of severe retinopathy after 20 years. However, no significant difference was reported in the prevalence of neuropathy, as assessed by monofilament testing, in the combined intervention group compared to the control group. However, monofilament testing is not the most sensitive measure for neuropathy.
The Diabetes Prevention Program (DPP) also demonstrated that a physical activity and dietary intervention can normalize features of metabolic syndrome and reduce the incidence of T2DM by 58% in people with impaired fasting glucose (IFG) or IGT. In fact, the DPP found that a lifestyle intervention was almost twice as effective as metformin in preventing conversion from IGT to diabetes. To prevent one case of diabetes in 3 years, 6.9 persons would have to participate in the lifestyle intervention program, compared to 13.9 people who would have to receive metformin . At the end of the DPP, all participants were offered the lifestyle intervention training. After 15 years of follow-up, there was evidence of the effects of metabolic memory . The incidence of diabetes was reduced by 27% in the original lifestyle intervention group compared to the placebo group. In terms of other outcome measures, the prevalence rates of the aggregate microvascular outcome did not differ between the treatment groups. However, when examining the cohort of participants who developed T2DM, the original lifestyle intervention group showed reductions in neuropathy, which was defined as loss of sensation to a 10-g monofilament, compared to the placebo and metformin groups . This finding suggests previous lifestyle intervention in people with IGT or IFG who convert to diabetes may reduce the prevalence of DPN. It is important to note that in addition to DPN being defined by monofilament testing, there were no baseline assessments of neuropathy included in the DPP, so incident DPN could not be evaluated.
The Impaired Glucose Tolerance Causes Neuropathy (IGTN) Study was a 12-month natural history study of 32 patients with either IGT or IFG and mild neuropathy at the time of enrollment . It was designed to examine the effects of a lifestyle intervention on DPN and specifically included measures of small fiber neuropathy. Participants were given general dietary and physical activity advice that was similar to the goals of the DPP lifestyle intervention group. Those individuals who lost weight and/or increased their physical activity were found to have significant improvements in the proximal thigh IENFD obtained from a 3-mm skin punch biopsy (increase of 1.4 fibers/mm) and foot sweat volume measured by quantitative sudomotor axon reflex. The improvements in IENFD and sweat volume, which are both small fiber nerve measurements, were associated with significant improvements in weight, glucose tolerance, and lipid profile. The increase in the thigh IENFD levels was clinically relevant in that it was significantly correlated with decreased neuropathic pain scores. Although there was no control group for comparison, these findings suggest that aggressive treatment of IGT with a lifestyle intervention might improve clinically relevant measures of neuropathy. It also highlights the importance of intervention during the earliest stages of impaired glucose regulation before the development of frank diabetes.
The effect of lifestyle interventions has also been examined in T2DM. The Look AHEAD (Action for Health in Diabetes) study enrolled over 5000 overweight or obese patients with T2DM and randomized them to either an intensive lifestyle intervention or diabetes education . Similar to the DPP, baseline measurements did not include assessments for neuropathy. The primary outcome measure was rate of cardiovascular events, which did not show a difference after 9.6 years when the intervention was stopped. However, participants were followed after the intervention was stopped and the prevalence of neuropathy was assessed by the MNSI. The lifestyle intervention group was found to have a lower increase in neuropathic symptoms compared to the standard care group and this in turn was associated with the amount of weight loss. However, there was no effect of the lifestyle intervention on the MNSI physical examination score with the exception of light touch sensation.
In contrast to the Look AHEAD study, which was designed to assess cardiovascular events and did not assess for DPN at enrollment, an Italian study aimed to assess the effects of exercise training on the development of DPN . This 4-year-long study enrolled 78 participants with either T1DM or T2DM and no signs or symptoms of DPN. The participants were randomized to either an intense aerobic exercise program (4 hours of treadmill walking weekly) or a control group with no intervention. There was no dietary intervention included in the protocol. The exercise group did not significantly change their BMI, waist circumference, or lipid profile. However, they did significantly improve their exercise capacity and there was improvement in the NCV for both the peroneal and sural nerves ( P <.001) and fewer participants in the exercise group developed an increased VPT ( P <.5). The percentage of diabetic participants who developed DPN, based on clinical evaluation, at the end of the study was significantly lower in the exercise group compared to the control group ( P <.05). These findings suggest that aerobic exercise training may be an effective prevention strategy for DPN in diabetic patients, but larger confirmatory studies are needed. It is also important to note that assessments of small fiber neuropathy were not included in this study.
Using measurements of small fiber neuropathy obtained from distal and proximal lower extremity skin biopsies (IENFD and branching) as one of the predetermined outcome measures, a pilot study from the University of Kansas examined the effect of an exercise program on DPN progression in 17 patients with T2DM and neuropathy . This small group of subjects participated in a 10-week aerobic and resistance exercise program. At the end of the study, there was no change in weight, but the participants significantly improved their HbA1c as well as neuropathic symptoms measured by the Michigan Neuropathy Symptom Inventory and pain levels on a visual analog scale. There was also a significant improvement seen on the epidermal nerve fiber branching (0.11 branch nodes per fiber; P =.008) on the proximal skin biopsy. There was also a nonsignificant improvement in the IENFD (1.68 fibers/mm; P =.09) at the proximal biopsy site . Considering that this pilot study was only 10 weeks long, this finding was consistent with greater improvements seen over 1 year in the IGTN trial .
Results from the IGTN natural history study and pilot data from the University of Kansas suggest that lifestyle interventions may exert an effect on small unmyelinated epidermal axons. Probably this is because this subset of neurons is not only more vulnerable to the toxic effects of metabolic derangements, but also has a higher regenerative capacity and is therefore more amenable to therapies. Researchers at the University of Utah examined this hypothesis by utilizing a chemical axotomy technique that utilizes topical capsaicin to assess epidermal reinnervation by skin biopsy and serial measurement of IENFD . To examine the effect of exercise on unmyelinated axons in T2DM, researchers randomized 100 patients with T2DM and no signs or symptoms of DPN to either a weekly resistance and aerobic exercise program ( n =60) or general health counseling ( n =40). After the 1-year intervention, participants in the exercise group had a significantly increased IENFD at the distal leg (increased by 1.5 fibers/mm) compared to control subjects (decreased by 0.1 fibers/mm; P =.03). This improvement was seen despite no significant changes in metabolic parameters, other than an improvement in HDL. Although it was not statistically significant, fewer subjects in the exercise program developed neuropathy, defined as an abnormal score on the Utah Early Neuropathy Scale, compared to those in the control group (5.7% vs 17%; P =.08). These results provide further support that IENFD is a sensitive and responsive biomarker for future clinical trials in DPN. They also suggest that exercise can prevent or even reverse nerve damage to unmyelinated axons in T2DM.
The Utah group also used the same capsaicin axotomy technique to examine the effects of exercise on cutaneous regenerative capacity in metabolic syndrome . Since nerve injury in DPN is thought to start prior to the onset of diabetes, successful prevention strategies may be needed during the earliest stages of glucose dysregulation. This study enrolled 32 patients with metabolic syndrome and 35 patients with T2DM without signs or symptoms of neuropathy. Participants underwent thigh capsaicin axotomy and their baseline distal leg IENFD and 30-day regeneration rate were assessed and compared. A subset of 36 patients (17 with metabolic syndrome) was then assigned to participate in a 6-month intensive exercise and lifestyle counseling program and the regeneration rate was reassessed in month 4. At baseline, distal leg IENFD was significantly reduced in both metabolic syndrome and T2DM groups and the 30-day regeneration rate was comparable in both groups. After 4 months of the lifestyle intervention, the 30-day reinnervation rate increased to 0.051 fibers/mm/day compared to the baseline rate of 0.072 fibers/mm/day ( P =.002). Participants who achieved improvements in more components of metabolic syndrome had a greater increase in their reinnervation rate ( P <.012). These results provide further evidence that metabolic syndrome is associated with a similar reduction in IENFD and cutaneous regenerative capacity than is seen in T2DM.
In general, patients with diabetes are encouraged to participate in regular physical exercise and supervised exercise programs are well tolerated . However, patients with proliferative nephropathy should avoid exercise that can cause hypertension and weight-bearing exercises should be performed with caution in patients with more advanced neuropathy and insensate feet due to risk of developing a diabetic foot ulcer. In particular, patients with autonomic neuropathy can have an increased risk of exercise-induced injury due to decreased cardiac responsiveness to exercise, impaired thermoregulation, orthostatic hypotension, and greater susceptibility to hypoglycemia. In addition, because CAN is an independent risk factor for the development of silent myocardial infarction and cardiovascular death, patients with CAN should be screened and possibly undergo an exercise stress test before starting an exercise program. Patients with autonomic dysfunction should also avoid exercising in hot or cold environments because of the risk of dehydration due to difficulty with thermoregulation. Finally, in patients with symptomatic orthostatic hypotension, recumbent exercises or water aerobics may be safer activities.
Bariatric surgery
Similar to intensive lifestyle intervention programs, bariatric surgery can also bring about sustained weight loss. While bariatric surgery can result in the remission T2DM, it is not known if it can be an effective treatment for DPN. The clinical trials of a lifestyle intervention in DPN described above have suggested that exercise without significant weight loss or change in metabolic parameters is sufficient to improve DPN. While improved glycemic control alone has not been convincingly shown to be an effective treatment for DPN, is normoglycemia together with weight loss through bariatric surgery able to achieve this goal? While there has not been a randomized control trial to address this question, several small uncontrolled studies have found low-grade evidence that bariatric surgery may improve measures of DPN.
Two prospective studies of patients with T2DM who underwent gastric bypass surgery found conflicting effects on measures of DPN. The first study found that participants with existing preoperative neuropathy had an improvement in their Neuropathy Symptom Score and Neuropathy Deficit Score 6 months postoperatively . Interestingly, this improvement was independent of improved glucose control. Significant limitations of this study include the lack of a control group and there was no masking of the intervention during assessment of outcome measures. Another small prospective case control study of patient with T2DM who underwent gastric bypass surgery found no change in nerve conduction studies 1 year after surgery . Neither of these two studies focused on possible prevention of DPN in patients with T2DM who underwent gastric bypass surgery. A large retrospective observational cohort of over 4500 participants with T2DM who underwent bariatric surgery found a 29% lower risk of incident microvascular disease in participants whose T2DM remitted compared to those who never remitted . Interestingly, retinopathy accounted for much of the incident microvascular disease and neuropathy had a very low incidence. It remains unknown if bariatric surgery can prevent or treat existing DPN, and one must also consider the risks of surgery that include subacute axonal neuropathy due to micronutrient deficiencies.
Dietary interventions
There is a lack of evidence from clinical trials in humans on the effect of diet on DPN. Evidence from animal studies is also lacking, but mice fed with a high-fat diet provide an animal model of obesity and insulin resistance that can be used to study metabolic syndrome and the development of DPN. In a dietary study of the effects of a ketogenic diet, mice fed with a ketogenic diet had improvement in neuropathy based on an increase in epidermal axon density compared to mice fed with a high-fat diet or mice fed with a high-fat diet and subjected to exercise . The ketogenic diet both prevented and reversed mechanical allodynia and preserved IENFD . Despite a high fat content in both the high-fat and ketogenic diets, the ketogenic diet appears to affect peripheral nerves differently and promote axon growth, but this requires further study.
There is considerable interest in the effect of lipid metabolism and chronic inflammation on the pathogenesis of DPN. Animal studies indicate that high-fat diets can cause neuropathy in diabetes and that withdrawing or manipulating a high-fat diet can reduce neuropathy . In humans, a nutritional intervention study that was targeted to improve essential fatty acid dysmetabolism in T1DM showed improvement in neuropathy . In this uncontrolled open-label trial, patients with T1DM and DPN were given supplementation with seal oil omega-3 polyunsaturated fatty acids. After 12 months of supplementation, the participants were found to have a 29% increase in corneal nerve fiber length but there was no improvement found in other measures of neuropathy. Despite there being no improvement in other neuropathy outcomes, there was no observed progression of clinical disease symptoms over the year-long study and there were no declines in small and large fiber sensory and functional measures. This lack of progression may be significant, but the study lacked a control group.
Multifactorial treatment
Hypertension plays a role in the microvascular dysfunction seen in diabetes through an unclear mechanism or interplay among several mechanisms. Overall, it is thought that hypertension exerts an effect on pathways that contribute to an overall state of oxidative stress, inflammation, and ultimately disruption of normal cellular functioning and alterations to the endoneurial vasculature. One of the pathways that is likely involved is the renin-angiotensin system. Angiotensin-converting enzyme (ACE) inhibitors improve vascular dysfunction and promote vasodilation by preventing the production of angiotensin II, which is a pressor, and preventing the breakdown of bradykinin, which is a vasodilator. ACE inhibitors have been shown to delay progression of both nephropathy and retinopathy in humans, and animal studies have found an improvement in measures of diabetic neuropathy . In humans, over 9000 individuals with IGT and cardiovascular disease or cardiovascular risk factors were randomized to treatment with either valsartan, an angiotensin-receptor blocker, or placebo in addition to lifestyle modification . The participants were followed for 5 years and the cumulative incidence of diabetes was 33.1% in the valsartan group compared with 36.8% in the placebo group (HR 0.86, CI 0.80–0.92; P <.001). There was no significant change in the incidence of cardiovascular outcomes between the two groups.
There have been several small studies that have examined the effects of ACE inhibitors on DPN. A small randomized, double-blind, placebo-controlled trial of diabetic patients with mild DPN found that treatment with trandolopril was associated with improvements in nerve conduction studies but not in the VPT, neuropathic symptoms, or deficits . There was also no improvement in autonomic function. Another ACE inhibitor, quinapril, has been shown to increase parasympathetic activity in DAN . A study of the effects of combination therapy with an ACE inhibitor and a third-generation dihydropyridine calcium channel blocker found a positive effect on DPN. Therapy with an ACE inhibitor (delapril) alone or in combination with a calcium channel blocker (manidipine) for 3 years was found to reduce progression of diabetic neuropathy in 200 patients with T2DM and hypertension . The odds ratio for combined therapy was 0.45 (0.24–0.87, P =.017) and for ACE inhibitors was 0.52 (0.27–0.99, P =.048). Although these results are encouraging, the true effects of ACE inhibitors on DPN remain unknown. Many diabetic patients require treatment of hypertension and ACE inhibitors are often a first-line treatment in this patient population.
Few studies have examined the effect of multifactorial cardiovascular risk intervention on DPN. The Steno 2 study was an open, parallel trial of an intensive multifactorial intervention versus standard care in patients with T2DM . The intervention was an aggressive, stepwise implementation of lifestyle and pharmacologic therapies (including renin-angiotensin system blockers) that focused on hypertension and lipid abnormalities as well as exercise, smoking cessation and glucose control. There was no effect from the intensive multifactorial intervention on the progression of relatively advanced somatic DPN as measured by vibration detection threshold, which is not a sensitive measure of neuropathy. However, there was a reduced risk of autonomic neuropathy in the intensive therapy group compared to those in the conventional therapy group.
In contrast, the ADDITION study examined the effect of an early multifactorial intervention in participants with newly diagnosed T2DM . This was done to see if the beneficial effects of the intensive multifactorial intervention utilized in the Steno 2 study on cardiovascular mortality might be amplified if implemented earlier in the course of T2DM when the risk factors are still modifiable. The ADDITION-Denmark study enrolled over 1500 patients with newly diagnosed T2DM and randomized them to intensive multifactorial treatment or routine care. After 6 years, there was no statistically significant difference in the prevalence of DPN. However, there was no baseline neuropathy assessments included at the time of enrollment and the two groups had little to no differences in glycemic or other metabolic measurements. The multicenter ADDITION-Europe study followed over 3000 patients from the original ADDITION trial for 5 years to determine the effect of the multifactorial treatment on microvascular complications . Compared to routine care, the intense multifactorial treatment in early T2DM did not significantly reduce the frequency of microvascular events at 5 years. DPN, which was assessed by questionnaire, was present in 4.9% of patients in the intensive care group and 5.9% of patients receiving routine care. Similar to the original ADDITION trial, there was a lack of baseline assessment of neuropathy and both the intervention and standard care groups achieved similar improvements in cardiovascular disease risk factors, which might account for the lack of differences in DPN prevalence between the two groups.
Treatment based on pathogenic concepts
The underlying pathogenesis of DPN involves various pathologic pathways (glucose entry into the polyol pathway, oxidative stress, advanced glycation end-product (AGE) formation, microvascular ischemia, or adipocyte derived toxicity) that are each a potential therapeutic target. Several of these targeted treatments have been evaluated through randomized clinical trials with varying levels of evidence, but in general unequivocal evidence from phase 3 trials is lacking. Targeted therapies are not ideal due to the multitude of mechanisms underlying the development and progression of DPN. However, they do have the advantage of being able to exert their effects despite patient noncompliance with a lifestyle intervention or the inability to maintain normoglycemia.
Alpha-lipoic acid
Alpha-lipoic acid (ALA) is a naturally occurring, dietary supplement with antioxidant properties and it is an essential cofactor for mitochondrial bioenergetic enzymes. It has been studied in clinical trials of DPN because oxidative stress is thought to be a major contributor to the pathogenesis of DPN. Several meta analyses suggest that ALA is an effective and safe drug for the treatment of symptomatic DPN . The SYDNEY 2 trial was a randomized, double-blind, placebo-controlled trial of different doses of once daily, oral doses of either 600, 1200, or 1800 mg ALA in patients with existing DPN . A statistically significant reduction in the Total Symptom Score (TSS) compared to placebo was seen for each dose of ALA after 5 weeks. The TSS decreased by 51% in the 600 mg group, 48% in the 1200 mg group, and 52% in the 1800 mg group compared to 32% in the placebo group ( P <.05 vs placebo). Significant improvements were also found in the Neuropathy Symptoms and Change score and a lower score was found for the Neuropathy Impairment Score (NIS). All three ALA groups had improvement in neuropathic symptoms and deficits; however, side-effects were greater with higher ALA doses, and thus the recommended dose of ALA is 600 mg once daily.
The long-term efficacy and safety of ALA 600 mg once daily in patients with mild to moderate DPN was evaluated in the NATHAN 1 trial. This multicentered, randomized, double-blind, parallel-group trial suggested that ALA may improve neuropathic deficits in the long term with good tolerability. The primary endpoint was a composite score of the NIS-lower limb (NIS-LL) and seven neurophysiological tests and a clinical responder was defined as someone with an improvement of at least 2 points on the NIS and NIS-LL. After 4 years of treatment, the rates of clinical responders were higher and the rates of progressors were lower with the ALA versus placebo ( P =.013 and P =.025 for the NIS and NIS-LL, respectively) . However, there was no significant difference in the primary endpoint and for this reason the study drug was not approved by the US FDA. Post hoc analysis of the NATHAN 1 trial found that improvement and prevention of progression of the NIS-LL with ALA versus placebo was predicted by higher age, lower BMI, male sex, normal blood pressure, history of cardiovascular disease, insulin treatment, longer duration of diabetes and neuropathy, and higher neuropathy stage . The authors concluded that better outcomes in neuropathic impairments with treatment of mild to moderate DPN with ALA 600 mg daily was predicted by optimal control of cardiovascular risk factors in patients with more severe neuropathy.
The effects of ALA on CAN in patients with T2DM were examined by the Deutsche Kardiale Autonome Neuropathie (DEKAN) Study . This randomized, double-blind, placebo-controlled multicenter trial assigned patients with T2DM and a reduced heart rate variability at baseline to treatment with 800 mg ALA orally or placebo. After 4 months of treatment, the ALA was well tolerated and there were small, but not statistically significant improvements in components of the cardiac autonomic spectral analysis in the ALA-treated patients compared to placebo.
Sirtuin 1 (SIRT1) and diabetic polyneuropathy
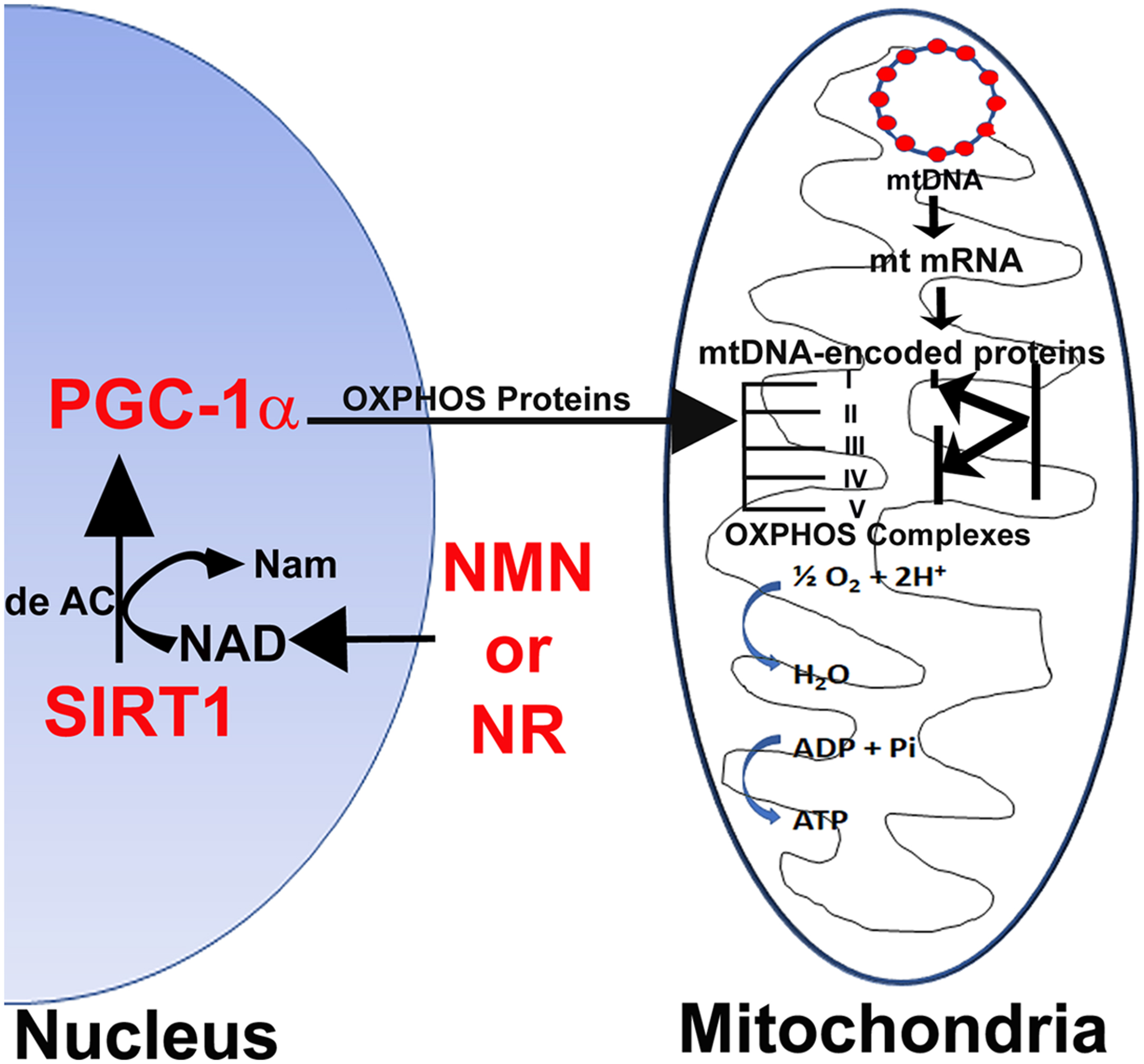
Activators of SIRT1 have been shown to prime the mitochondrion by increasing its reserve capacity to combat mitochondrial stress under diabetic conditions . Recent research has shown that SIRT1 is decreased in diabetic dorsal root ganglion neurons and overexpression can increase axonal outgrowth in the peripheral nerve . SIRT1 promotion of axonal repair would be critical in repairing degenerating axons in diabetic neuropathy. Neuronal overexpression of SIRT1 enhances the SIRT1/PGC-1α axis in neurons and protects against DPN. SIRT1 is upregulated by the NAD + precursors nicotinamide mononucleotide (NMN) or nicotinamide riboside (NR; Fig. 16.3 ) and is able to prevent and reverse DPN through improving Mt oxidative metabolism .
Role of NAD + analogs in diabetic polyneuropathy
The precursors of NAD + are nicotinamide (NAM), nicotinic acid (NA), NR, and NMN. The reduction in NAD + induced in diabetes is due to increased DNA damage, stimulating PARP1 activity . Long-term treatment with NAM is detrimental because they increase development of a fatty liver, due to reductions in available methyl groups . NAD + cannot be given directly because of its toxic effects that include serious hyperglycemia lasting for hours. NA often causes severe flushing, mediated by the binding of NA to the GPR109A receptor . NAD + precursors that do not activate GPR109A , for example NR and NMN, but still increase NAD + levels are more likely to be clinically useful. The dietary supplement NR is a major generator of NAD + , which activates neuronal protection and regeneration pathways . NR is well tolerated and orally bioavailable and protects against both sensory and motor neuropathy in animals . NR and NMN function through numerous pathways. However, one of the primary pathways is to replete NAD + , thereby deacetylating SIRT1 that in turn activates PGC-1 and improves mitochondrial function in diabetic neuropathy ( Fig. 16.3 ).
Benfotiamine
Benfotiamine is a lipid-soluble derivative of vitamin B1 that blocks several hyperglycemia-induced pathways that are implicated in the development of hyperglycemia-induced vascular damage: the hexosamine pathway, the AGE formation pathway, the diacylglycerol-protein kinase C pathway, and NF-kappa B activation by activating the pentose phosphate pathway enzyme transketolase . Benfotiamine has been shown to increase levels of intracellular thiamine and reduce AGEs that induce experimental diabetic neuropathy . The BEDIP study, a pilot study in early DPN of benfotiamine 100 mg four times a day for 3 weeks, found a statistically significant improvement in a neuropathy score that included evaluation of patients’ symptoms, VPT, and exam findings of neuropathy . The follow-up double-blind, placebo-controlled, phase III BENDIP study randomized 165 patients with DPN to 6 weeks of treatment with benfotiamine 600 mg a day, benfotiamine 300 mg/day, or placebo . The 600 mg/day dose group had a small improvement in the primary outcome parameter, the Neuropathy Symptom Score, compared to placebo in the per-protocol group, but no significant difference was seen in the intention to treat population ( P =.055). A 24-month trial of 300 mg/day benfotiamine compared to placebo in patients with T1DM found no significant difference in peripheral nerve function or soluble markers of inflammation . However, only a few of the enrolled participants had neuropathy or inflammation at baseline.
Actovegin
Actovegin is a deproteinized hemoderivative that is extracted from calf blood by ultrafiltration. It is made up of several hundred bioactive substances that exert an effect on many intracellular processes and its neuroprotective effects are thought to be due in part to its ability to stimulate oxygen uptake and consumption. In addition, actovegin has been shown to have an insulin-like effect and results in increased glucose oxidation . In animal models of diabetes, actovegin has been found to improve DPN through suppression of poly(ADP-ribose) polymerase activation . A clinical trial in humans randomized 567 patients with DPN to receive 20 daily infusions (2000 mg/day) followed by three tablets (1800 mg/day) a day of actovegin or placebo for 140 days . After 160 days, there was a significant improvement seen in the two primary outcome measures, the TSS of the lower limbs and VPT in the actovegin group compared to placebo. There was also a significant improvement in the sensory nerve function component of the NIS-LL. There were no differences in the incidence of adverse events between the groups. While these results are promising, long-term, confirmatory trials with inclusion of objective neuropathy outcome measures are needed. In addition, the mechanism of action of actovegin is still not elucidated and further investigations into the mode of action with in vitro and in vivo studies are needed.
Epalrestat
Epalrestat is an aldose reductase inhibitor that is marketed in Japan, China, and India for the treatment of DPN. Aldose reductase inhibitors were a promising therapeutic agent in DPN due to their effects on the polyol pathway, but clinical data from randomized control trials in DPN is lacking. Aldose reductase is a key enzyme of the polyol pathway, which in enhanced in the development of DPN, and its inhibition was hypothesized to be a key component in the treatment of DPN. Numerous clinical trials with aldose reductase inhibitors have been carried out but none of them have been approved in the United States due to poor clinical trial designs, lack of a control group, limited efficacy, or unacceptable adverse events. One open-label, controlled trial showed a small but significant change from baseline in motor NCV in patients with DPN with epalrestat compared to control after 3 years .
Another aldose reductase inhibitor, ranirestat, has also been examined for its effect on measures of DPN. A clinical trial of patients with DPN randomized 549 participants with mild to moderate DPN to placebo or 10, 20, or 40 mg/day of ranirestat for a year . Treatment with ranirestat was associated with an improvement in motor nerve function on nerve conduction studies, but there was no statistically significant difference in sensory nerve function compared to placebo. There was also no improvement seen in the modified Toronto Clinical Neuropathy Score (mTCNS) or quantitative sensory testing. A more recent double-blind, placebo-controlled, phase III study of 557 participants with DPN found a significant increase in tibial motor NCV (0.52 m/s, P =.021), the median motor nerves, proximal median sensory nerves, and distal median sensory nerves in those treated with ranirestat 40 mg/day compared to placebo after 1 year . No significant differences were found in the mTCNS between the two groups. Ranirestat was well tolerated.
Antiinflammatory drugs
Subclinical inflammation is a characteristic of T1DM and T2DM and there is a growing body of evidence that low-grade, chronic inflammation plays an important role in the pathogenesis of DPN and may be a potential therapeutic target. Hyperglycemia, along with insulin resistance and dyslipidemia, leads to systemic inflammation and a cycle of oxidative and mitochondrial stress that causes cellular damage and perpetuates the cycle. Multiple factors are involved in this cascade that ultimately produces cytokines and chemokines that enhance inflammation. The result of the inflammation is an increase in downstream oxidative stress and further neuronal damage. A full review of inflammation in diabetic neuropathy is outside the scope of this chapter, but the role of chronic inflammation as a potential therapeutic target in DPN will be discussed.
A link between diabetes and inflammation was first suggested by epidemiological studies that found a correlation between T2DM and inflammatory markers such as fibrinogen, C-reactive protein, interleukin-6, plasminogen activator inhibitor-1, and sialic acid . More concrete evidence for the role of inflammation in T2DM came from the finding that salsalate, a salicylate prodrug, improved glycemic control and decreased inflammatory markers in T2DM . Salicylate acts on the nuclear factor-kB (NF-kB) pathway, which has been found to be a key pathway in the inflammatory response seen in diabetic nerves . The NF-kB pathway is activated by hyperglycemia, oxidative stress, and cytokines and goes on to trigger an inflammatory response that not only results in direct cellular injury, but it also regulates the expression of many inflammatory genes (COX-2, NO-synthase, lipoxygenase, and endothelin-1) . The NF-kB pathway also regulates the expression of cytokines, including tumor necrosis factor-α (TNF-α). Activation of TNF-α activates mitogen-activated protein kinase , which is found in association with DPN. Furthermore, inactivation of the COX-2 gene has been found to prevent peripheral nerve dysfunction .
Observations in human DPN mirror those in animals. In DPN, compared to diabetics without neuropathy and control subjects, there is an increase in serum levels of inflammatory cytokines, including TNF-α . In addition to the increase in TNF-α in DPN, levels of the antiinflammatory cytokine IL-10 were reduced . Similarly, a population-based study found that serum levels of several inflammatory cytokines, including IL-1β and IL-6, were associated with DPN . Further evidence that cytokines may be a potential target for the treatment of DPN is the finding that higher levels of IL-1 receptor antagonist (IL-1RA) are associated with incident DPN and the progression of DPN .
Dysregulation of heat shock proteins (HSPs), which chaperone cells and protect them from environmental stress, has also been found to be associated with DPN. In humans, diabetes has been shown to decrease the expression of HSP70 and HSP 27, and increasing the expression of HSP70 can improve DPN . In animal models, mice overexpressing HSP27 were less likely to develop DPN. This reduced incidence of neuropathy was correlated with a decrease of NF-kB activation . In humans, however, a cross-sectional study of patients with T1DM found that higher levels of HSP27 correlated with the presence of DPN . An explanation for this finding may be that the increased levels of HSP27 are a result of a secondary neuroprotective response to the developing DSP .
There is strong support for the role of inflammation early in the development of both diabetes and DPN. Together with insulin resistance and adiposity, inflammation contributes to ongoing metabolic dysfunction and cytotoxicity that results in peripheral nerve dysfunction. Thus targeting inflammation is a potential therapy for DPN.
Conclusion
For DPN, the most effective interventions are improved glycemic control in T1DM and lifestyle interventions in T2DM. Several medications show promise in modifying DPN but require confirmation with large phase 3 trials. In future trials, the endpoints need to be carefully selected to ensure adequate sensitivity to change; therapies should be introduced earlier in the course of DPN to avoid expensive, extended duration studies; drugs need to target pathogenetic mechanisms; and therapies may be more effective in combination because they target different pathogenic mechanisms.
Acknowledgments
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health 1R01DK107007–01A1, Office of Research Development, Department of Veterans Affairs (Biomedical and Laboratory Research Service and Rehabilitation Research and Development, 101RX001030), Diabetes Action Research and Education Foundation, University of Maryland Institute for Clinical & Translational Research (ICTR), and the Baltimore GRECC (JWR), 1K2RX001651 (LAZ).
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree