Diseases of the central nervous system (CNS) are common in patients with Langerhans cell histiocytosis (LCH). Besides active LCH lesions, neurodegenerative (ND) lesions of the cerebellum and/or basal ganglia may occur as late sequelae of LCH. While the etiology of this ND disease remains unclear, biomarkers in cerebrospinal fluid (CSF) may reflect the activity of CNS disease in these patients. However, no well-planned CSF studies have yet been performed in patients at high risk for ND-CNS-LCH. Potential parallels with other neuroinflammatory/neurodegenerative disease suggest the utility of examining these other disorders in establishing strategies for the prevention and/or treatment of ND-CNS-LCH.
Key points
- •
The syndrome of neurodegeneration-central nervous system-Langerhans cell histiocytosis (ND-CNS-LCH) in a subset of patients with LCH remains a progressive and devastating complication. Although a definitive incidence of this clinical syndrome remains unclear, estimates suggest around 10%. Patients at high risk for developing ND-CNS-LCH usually have disease involvement of the mastoid, temporal, and orbital bones as well as having developed diabetes insipidus.
- •
ND-CNS-LCH is usually a waxing and waning, yet progressive, disorder characterized by radiographic involvement of the cerebellar peduncles, basal ganglia, and often pons. Clinical signs and symptoms include problems with physical coordination (ataxia, dysarthria, dysmetria) as well as neurocognitive and psychological difficulties.
- •
The cause of ND-CNS-LCH is unknown, but seems to be in part mediated by CD8-positive lymphocytes and neuroinflammatory cytokines/chemokines. Whether ND-CNS-LCH is due to the presence of active, yet undetectable by current methods, LCH, or a paraneoplastic consequence of dendritic cell activation of the immune system to recognize CNS antigens is unknown.
- •
Several attempts at treatment with immunosuppressive or cytotoxic/immunosuppressive approaches have not resulted in an optimal strategy. There is a great need for prospective, randomized trials that will also measure critical biological and clinical characteristics of patients with new onset ND-CNS-LCH as well as for newly diagnosed patients with LCH at high risk for developing ND-CNS-LCH.
Introduction
Langerhans cell histiocytosis (LCH) is a myeloid precursor/dendritic-cell-related histiocytosis; approximately two-thirds of those affected are children. LCH is characterized by mutations of genes of the ERK signaling pathway as well as a lesional “cytokine storm,” a term referring to both the high level and the diversity of locally synthesized cytokines. Thus, in many ways and like many other neoplastic disorders, LCH can be considered an inflammatory neoplasia, meaning that although proliferation and survival of the neoplastic cells are driven by key genetic changes, the pathophysiology of the neoplasia is modified by the interchange of the neoplastic cells with their microenvironment and vice versa. Thus, increased levels of several of a variety of cytokines/chemokines have been found in the plasma/serum of patients with active LCH, and these levels typically reflect systemic disease activity ( Table 1 ).
| Investigated Biological Materials | Cytokines/Chemokines Involved | References |
|---|---|---|
| Lesional | IL-1, IL-6, M-CSF 2 , GM-CSF 2 , TNFα, IL-3, CD40L, RANKL-RANL CCR6-CCL20, CCR7, CCL5, CXCR3-CXCL11 | |
| Systemic (serum/plasma) | IL-1RA, TNFα, IL-1β, CD40L, RANK, RANKL, OPG, sIL-2R | |
| CSF 1 (cerebrospinal fluid) |
| |
| ||
| Needs to be studied |
Central nervous system (CNS) lesions are also common in LCH. These lesions include active lesions (ie, clear involvement with lesional LCH cells) involving the hypothalamic-pituitary axis with secondary central diabetes insipidus (CDI), space-occupying lesions at other sites, and neurodegenerative (ND) lesions of the cerebellum and basal ganglia. Patients with an increased risk for the development of CNS lesions have been defined among patients with LCH. Besides permanent CDI, the development of ND-CNS-LCH represents the most serious late CNS sequela.
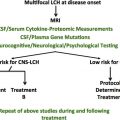
One of the hypotheses regarding CNS neurodegenerative disease (NDD) is that the level of CNS lesional cytokines/chemokines at disease onset may contribute to the subsequent development of CDI and ND-CNS-LCH disease, and that this level will be reflected in the cerebrospinal fluid (CSF). This hypothesis is based on consideration of relevant studies on serum/plasma measurements reflecting systemic disease activity. To date, there are reports concerning cytologic findings in CNS-LCH ; however, only one CSF study has been performed to determine cytokines/chemokines activity within the CNS in patients with ND-CNS-LCH.
The paucity of such data for patients with LCH, in contrast to leukemia and lymphoma, is in part due to the fact that lumbar puncture is not a routine procedure in the management of LCH. Thus, lumbar punctures would need to be considered as research studies at this time for patients with LCH, and this has not routinely been incorporated into prospective trials for these patients. The determination of such cytokine/chemokine profiles as well as determination of the cell types, gene mutation determination, and proteomic analysis at the time of diagnosis in at least all high-risk patients would be potentially worthwhile to identify possible predictive biomarkers at disease onset and at times of reactivation as well as when CNS NDD radiographic or clinical findings are noted.
Inflammatory processes commonly play an important role in the pathway that leads to neuronal cell death in many NDDs ( Fig. 1 ). The inflammatory response usually involves the perivascular area. Here, invading T cells interact with the activated microglia, which are resident immune cells of the CNS and serve as a pathologic hallmark of inflammatory brain disease. Chronic activation of the microglia may result in neuronal damage because of the release of cytotoxic molecules, such as proinflammatory cytokines, reactive oxygen intermediates, proteinases, and complement proteins. The suppression of microglia-mediated inflammation may thus represent an important prophylactic or therapeutic strategy for the management of ND-CNS-LCH disease. The facilitation of neuroprotection may also be important, such as through the use of agents that target the trafficking of blood cells into the CNS.
This review presents data on findings regarding LCH-related CNS findings, including the CSF profiles of cytokines/chemokines and other molecular factors in a range of neurologic diseases. Second, it discusses which CSF analyses may be indicated for study in patients with LCH, and the development of clinical trials to test strategies for the prevention of CNS-LCH lesions. The overall goal is to demonstrate a conceptual framework in which to further develop optimal therapeutic strategies for patients at high risk for the development of CNS-LCH.
Introduction
Langerhans cell histiocytosis (LCH) is a myeloid precursor/dendritic-cell-related histiocytosis; approximately two-thirds of those affected are children. LCH is characterized by mutations of genes of the ERK signaling pathway as well as a lesional “cytokine storm,” a term referring to both the high level and the diversity of locally synthesized cytokines. Thus, in many ways and like many other neoplastic disorders, LCH can be considered an inflammatory neoplasia, meaning that although proliferation and survival of the neoplastic cells are driven by key genetic changes, the pathophysiology of the neoplasia is modified by the interchange of the neoplastic cells with their microenvironment and vice versa. Thus, increased levels of several of a variety of cytokines/chemokines have been found in the plasma/serum of patients with active LCH, and these levels typically reflect systemic disease activity ( Table 1 ).
| Investigated Biological Materials | Cytokines/Chemokines Involved | References |
|---|---|---|
| Lesional | IL-1, IL-6, M-CSF 2 , GM-CSF 2 , TNFα, IL-3, CD40L, RANKL-RANL CCR6-CCL20, CCR7, CCL5, CXCR3-CXCL11 | |
| Systemic (serum/plasma) | IL-1RA, TNFα, IL-1β, CD40L, RANK, RANKL, OPG, sIL-2R | |
| CSF 1 (cerebrospinal fluid) |
| |
| ||
| Needs to be studied |
Central nervous system (CNS) lesions are also common in LCH. These lesions include active lesions (ie, clear involvement with lesional LCH cells) involving the hypothalamic-pituitary axis with secondary central diabetes insipidus (CDI), space-occupying lesions at other sites, and neurodegenerative (ND) lesions of the cerebellum and basal ganglia. Patients with an increased risk for the development of CNS lesions have been defined among patients with LCH. Besides permanent CDI, the development of ND-CNS-LCH represents the most serious late CNS sequela.
One of the hypotheses regarding CNS neurodegenerative disease (NDD) is that the level of CNS lesional cytokines/chemokines at disease onset may contribute to the subsequent development of CDI and ND-CNS-LCH disease, and that this level will be reflected in the cerebrospinal fluid (CSF). This hypothesis is based on consideration of relevant studies on serum/plasma measurements reflecting systemic disease activity. To date, there are reports concerning cytologic findings in CNS-LCH ; however, only one CSF study has been performed to determine cytokines/chemokines activity within the CNS in patients with ND-CNS-LCH.
The paucity of such data for patients with LCH, in contrast to leukemia and lymphoma, is in part due to the fact that lumbar puncture is not a routine procedure in the management of LCH. Thus, lumbar punctures would need to be considered as research studies at this time for patients with LCH, and this has not routinely been incorporated into prospective trials for these patients. The determination of such cytokine/chemokine profiles as well as determination of the cell types, gene mutation determination, and proteomic analysis at the time of diagnosis in at least all high-risk patients would be potentially worthwhile to identify possible predictive biomarkers at disease onset and at times of reactivation as well as when CNS NDD radiographic or clinical findings are noted.
Inflammatory processes commonly play an important role in the pathway that leads to neuronal cell death in many NDDs ( Fig. 1 ). The inflammatory response usually involves the perivascular area. Here, invading T cells interact with the activated microglia, which are resident immune cells of the CNS and serve as a pathologic hallmark of inflammatory brain disease. Chronic activation of the microglia may result in neuronal damage because of the release of cytotoxic molecules, such as proinflammatory cytokines, reactive oxygen intermediates, proteinases, and complement proteins. The suppression of microglia-mediated inflammation may thus represent an important prophylactic or therapeutic strategy for the management of ND-CNS-LCH disease. The facilitation of neuroprotection may also be important, such as through the use of agents that target the trafficking of blood cells into the CNS.
This review presents data on findings regarding LCH-related CNS findings, including the CSF profiles of cytokines/chemokines and other molecular factors in a range of neurologic diseases. Second, it discusses which CSF analyses may be indicated for study in patients with LCH, and the development of clinical trials to test strategies for the prevention of CNS-LCH lesions. The overall goal is to demonstrate a conceptual framework in which to further develop optimal therapeutic strategies for patients at high risk for the development of CNS-LCH.
Laboratory studies of relevance to central nervous system-Langerhans cell histiocytosis disease
Cytokines/Chemokines and Other Cerebrospinal Fluid Markers in Neuroinflammatory Diseases
Most of the cytokines/chemokines that have been tested in the CSF of patients with neuroinflammatory disease have been proinflammatory cytokines (interleukin [IL]-1β, IL-6, and IL-8) and chemokines associated with Th1 CXC chemokine ligand (CXCL9-11), Th2 (CCL22), and B cells (CXCL13) (see Table 1 ). Chemokines and their receptors are involved in T-cell trafficking into the CNS. In particular, the chemokine CXCL10 regulates the migration of activated T cells into the CNS by binding to the CXC chemokine receptor (CXCR3). Increased levels of CCL5, CXCL10, CXCL12, and CCL19 in the CSF of patients with neuroinflammation have been reported. Osteopontin (Opn), a cytokine with a pivotal role in cellular immune responses, has been found to be elevated in the CSF of patients with a variety of neuroinflammatory diseases. In contrast, although high CSF concentrations of the decoy receptor 3 (DcR3) have been found in a range of neurologic diseases, it was barely detectable in the serum of affected patients. DcR3 is a member of the TNF receptor superfamily and was initially thought to inhibit the cytokine responses of FasL. DcR3 has also been shown to induce the formation of osteoclasts from human monocytes.
High CSF levels of IL-6, IL-12, IL-1β, and tumor necrosis factor-α (TNF-α) distinguish bacterial from aseptic meningitis. IL-6, IL-8, and TNF-α in CSF are particularly important mediators of the meningeal inflammatory process. Increased CSF concentrations of TNF-α, IL-1-α, and IL-6 have also been reported in pediatric patients with acute encephalitis/encephalopathy. Increased CSF levels of TNF-α, IL-1ra, and CXCL8 have been demonstrated in patients with malaria. In patients with tick-borne encephalitis, the detection of increased CSF concentration of CXCL10 suggests that this chemokine may play a role in the recruitment of CXCR3-expressing T cells into the CNS. Increased CSF levels of CXCL10 in subacute sclerosing panencephalitis, and of CXCL11 in acute neuroborreliosis, have also been reported. High CSF levels of Opn have been reported in patients with HIV-associated dementia.
In patients with multiple sclerosis (MS), elevated IL-1β in CSF is a predictive biomarker for active disease. Significantly increased CSF concentrations of CXCL10 have also been reported, whereas the CSF concentrations of CCL2 were found to be reduced in MS, compared with those in control groups. The levels of CXCL8 and CCL2 were found to be higher in CFS than in serum, and significantly increased levels of CXCL8 and CCL5 were observed during relapse. In MS, it has been hypothesized that CXCL13 plays a major role in the CNS recruitment of B cells and T cells that express the chemokine receptor CXCR5; these molecules are thus potential therapeutic targets. Mellergård and colleagues demonstrated a decrease in the CSF concentration of CXCL13 in patients with MS who had received high-dose methylprednisolone and natalizumab. High CSF concentrations of Opn have also been reported in MS.
Increased CSF levels of CCL2 and CXCL10 have been reported in Guillain-Barre syndrome, and increased levels of macrophage inflammatory protein-3 beta (MIP-3β) and interferon gamma inducible protein 10 (IP-10) have been reported in chronic inflammatory demyelinating polyradiculoneuropathy. DcR3 has been shown to be a potential suppressor of experimental autoimmune encephalitis in experiments involving its intrathecal administration in mice. In one patient with neuro-Sweet disease, CSF concentrations of IL-6, interferon-γ (IFN-γ), CXCL8, and CXCL10 were found to be substantially higher than in control subjects with other neurologic diseases. Increased CSF levels of IL-6 have also been reported in neuro-Behçet syndrome.
Most NDDs of the CNS are associated with microglia-mediated inflammation, and CD40 signaling plays a critical role in this process. Increased activation of the CD40-CD40L complex is found in major forms of dementia, and this leads to the increased synthesis of proinflammatory cytokines by immune cells in the CNS. Of interest, similarly high serum concentrations of soluble CD40L (sCD154) have been demonstrated in patients with multifocal LCH. Measurement of the concentration of soluble CD40L (sCD154) in CSF may thus be worthwhile to investigate in patients with LCH.
Increased soluble TNF is a hallmark of acute and chronic neuroinflammation as well as of many NDDs. The CSF concentrations of MCP-1 and IL-8 were found to be significantly higher in patients with amyotrophic lateral sclerosis than in controls. In postpolio syndrome (PPS), high CSF levels of IFN-γ, TNF-α mRNA, IL-2, and sIL-2R have been reported.
In multiple myeloma (MM), DcR3 is synthesized by myeloma cells and then interacts with FasL, a process that has been found to inhibit osteoclast apoptosis, which may, in turn, contribute to the osteolytic aspect of myeloma. However, the level of DcR3 in the CSF has not yet been established in MM. In patients with CNS lymphoma, Fischer and colleagues assessed levels of CXCL12 and CXCL13 in both CSF and serum and found that the CSF concentration of CXCL13 was correlated with the degree of blood-brain barrier (BBB) disruption. Patients with CNS lymphoma have also been shown to have high CSF concentrations of soluble CD27/DPP4 (dipeptidyl peptidase-4), an enzyme with known immunostimulating activity.
Matrix Metalloproteinases and Other Cerebrospinal Fluid Molecules in Neuroinflammatory Diseases
Extracellular matrix glycoproteins are components of cell-cell boundaries within the microvascular and extravascular tissues of the CNS. Matrix metalloproteinases (MMPs) are involved in the migration of leukocytes into the CNS during inflammation. Release of MMPs into the CSF may indicate CNS inflammatory disease or disruption of the BBB following an ischemic cerebrovascular accident. Activated microglia are known to secrete MMP. Soluble forms of vascular cell adhesion molecule-1, intracellular adhesion molecule-1 (ICAM-1), and E-Selectin play key roles in the response to BBB injury and are considered biomarkers of disease activity in MS. The CSF concentrations of the nerve/glial cell marker proteins, such as glial fibrillary acidic protein (GFAP), light subunit of neurofilament protein (NFL), and neuron-specific enolase (NSE), have been assayed in various neuroinjury and ND disorders ( Table 2 ). In Lyme neuroborreliosis with meningoradiculitis, the pretreatment CSF levels of GFAP and NFL were correlated with clinical outcome, and they declined significantly in response to treatment.
| Category | Disease | Increased Cytokines/Chemokines in CSF |
|---|---|---|
| CNS infections | Meningitis (bacterial, aseptic) | IL-6, IL-1β, IL-8, TNF-α, IL-12 |
| Encephalitis/encephalopathy | TNF-α, IL-1β, IL-6, IL-10, sTNFR1 | |
| SSPE | IL-12, CXCL10 | |
| Lyme neuroborreliosis | GFAP, NFL | |
| Autoimmune diseases | Multiple sclerosis | IL-1Ra, IL-1RII CCL2, CCL5, CXCL10, CXCL8, DcR3 CXCL13 |
| Neuro-Sweet disease | IL-6, IFN-γ, CXCL8, CXCL10 | |
| Neuro-Behçet disease | MMP-9, TIMP-1 | |
| NDDs | AD, PD | Tau-T, Aβ1-42, P-tau181P, |
| ALS | MCP-1, IL-8 | |
| PPS | IL-2, sIL-2R, mRNA of IFN-γ, TNFα | |
| Autoantibody-related CNS disease | CDI, Sydenham chorea, encephalitis | AVPc-Abs, anti-basal ganglia-Abs, anti-NMDAR-Abs |
| Hemato-oncology | CNS lymphoma | CXCL13, sCD27, sIL-2R |
| MM | DcR3 | |
| BBB disruption | Ischemia | MMP-9 |
| Brain injury | ALL-related | NSE, GFAP, NFL, AsR |
| Other | MBP, GFAP, NCAM, NSE | |
| Astrogial/neuronal interaction | GFAP, NFL |
Increased CSF levels of MMP-9 have been reported in viral, bacterial, and fungal meningitis; the concentrations of tissue inhibitor of metalloproteinases (TIMP-1) were found to greatly exceed those of MMP-9. MMPs are thought to be involved in demyelination and the disruption of the BBB in MS. MMP-9, in particular, is considered a marker of MS pathogenesis. Active MMP-9 and the MMP-9/TIMP-1 ratio have been found to be increased in the CSF of patients with MS. The MMP-9/TIMP-1 ratio was also found to be significantly increased in neuro-Behçet disease, and polymorphonuclear cells were identified as one source of the MMP-9 that was detected in the CSF. Because the serum concentrations of MMP and TIMP-1 are much higher than those in the CSF, it has been hypothesized that the serum active MMP-9/TIMP-1 ratio may be a useful surrogate marker for the monitoring of neuroinflammatory disease activity. Mitosek-Szewczyk and colleagues showed reduction of concentrations of soluble intercellular adhesion molecule-1 (sICAM-1) and sE-Selectin in serum, but not in CSF, after cladribine therapy in patients with remitting-relapsing MS.
The NSE level in CSF is of prognostic value in acute cerebellar ataxia. High CSF levels of NSE, GFAP, and NFL have been reported in patients with acute lymphocytic leukemia, and it has been suggested that these increases can be interpreted as early signs of brain injury. Gonzalez and colleagues performed proteomic profile studies in PPS and identified novel candidate protein biomarkers. Other CSF biomarkers of neurodegeneration, carbonyl proteins, and leucine-rich α2-glycoprotein have been described. In ND-CNS-LCH, Gavhed and colleagues reported increased levels of NFL, tau protein, and GFAP in a subset of their patient sample. Of these, the patients with most severe clinical and neuroradiological signs displayed the highest levels of NFL and GFAP.
Two types of NDD are due to an inborn error of metabolism. One is brain iron accumulation, which is caused by mutations of the pantothenate kinase 2 ( PANK2 ) gene, and the other is infantile neuroaxonal dystrophy, which is caused by mutations in the gene encoding phospholipase A(2) group VI ( PLA2G6 ). No CSF studies have been reported for these diseases to date.
Autoantibodies and Neuroinflammation
The role of autoantibodies in the development of ND-CNS-LCH disease remains unknown. One report detected no autoantibodies to CNS tissue in patients with ND-CNS-LCH. However, there are some clear parallels between various paraneoplastic autoimmune syndromes that are due to antineuronal autoantibodies and the paraneoplastic nature of ND-CNS-LCH. Autoantibodies have also been identified for CDI and for disorders of the basal ganglia. Maghnie and colleagues reported the presence of circulating anti-vasopressin-cell antibodies (AVPc-Abs) in 9 patients with idiopathic CDI, 4 patients with LCH, and 2 patients with germinoma. Pivonello and colleagues cautioned that AVPc-Abs may mask LCH or germinoma, and thus a diagnosis of CDI secondary to LCH must be assigned with caution. Antibasal ganglia antibodies have been detected in Sydenham chorea and other poststreptococcal neuropsychiatric disorders. Anti-NMDAR ( N -methyl- d -aspartate receptor) paraneoplastic encephalitis has been described and usually presents with disturbances of memory, behavior, and cognition. Such findings suggest that studies are warranted to determine if autoantibodies of relevance to the development of ND-CNS-LCH disease can be observed in a consistent fashion in patients with new onset disease.
Complement Activation Cerebrospinal Fluid Markers in Neuroinflammatory and Neurodegenerative Diseases
CNS damage in neurologic disorders such as neuromyelitis optica (NMO) and myasthenia gravis has been shown to be in part mediated by the complement system. In these diseases, anti-APQ4 or anti-AChR autoantibodies can mediate complement-dependent cytotoxicity. Of various complement proteins (C3a, C4a, C5a, and sC5b-9) produced in complement activation, the C5a levels in CSF were elevated significantly and correlated with the severity of exacerbation in NMO patients. Also, CSF levels of sC5b-9 are increased in patients with NMO, reflecting the activation of complement in the disease. For such diseases, antibody-based C5 inhibitor eculizumab has been suggested to be of potential benefit as treatment. In the pathogenesis of NDDs, the activation of the complement system was also proposed to be involved. For instance, in Parkinson disease (PD), CSF levels of complement 3 and factor H were shown to predict the disease activity.
Cerebrospinal Fluid Biomarkers in Alzheimer Disease and Parkinson Disease
Alzheimer disease (AD) and PD are the most common ND disorders. In AD, extensive studies have been pursued to develop therapy that could slow or arrest disease progression. CSF biomarkers have been extensively studied in AD and PD. Particularly in AD, CSF biomarkers, such as total tau (tau-T), β-amyloid 1-42 (Aβ1-42), phosphorylated tau at threonine 181 (P-tau181P), are used in clinical practice in diagnosis and as markers for evaluating disease modification. Investigators have found that abnormalities in CSF markers are associated with the development of mild cognitive impairment. In PD, besides the above-mentioned markers, determination of NFL in CSF is said to be useful in the differential diagnosis between PD and other Parkinsonian syndromes. These types of studies may help provide the basis for further investigation of CSF levels in LCH and early onset of ND-CNS-LCH.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree