Fig. 10.1
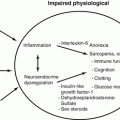
Treatment of malignant sex cord-stromal tumors. *Fertility-preserving surgery—affected-side salpingo-oophorectomy + omentectomy + peritoneal cytology + detailed intra-abdominal examination. **Lymph node dissection (biopsy) can be omitted. Reprint with permission from ref. 21
10.3 Malignant Ovarian Germ Cell Tumors (MOGCTs)
10.3.1 Clinical Features of MOGCTs
In Japanese population, the MOGCTs account for 3–4% of malignant ovarian neoplasia [2–4] and have very characteristic clinical features. Firstly, they represent 80% of preadolescent ovarian malignancies. Secondly, they have excellent sensitivity to chemotherapy. Thirdly, most cases show unilateral occurrence. These features permit the possibility of fertility-sparing treatment in such patients.
Malignant transformation in ovarian mature cystic teratoma is the most frequent type of MOGCT, accounting for 38% of MOGCT patients in Japan, while yolk sac tumors, dysgerminomas, and immature teratomas account for 23%, 17%, and 11%, respectively. Grading of immature teratoma is a recent important issue, with these tumors having been graded from 1 to 3, depending on the amount of immature neuroectodermal component in tissue specimens [22]. Recently, however, a two-tiered (low- and high-grade) system has been more commonly used [23]. In this new system, Grade 1 is categorized as low grade, while Grades 2 and 3 are classified as high grade. The latter is considered as an indication for chemotherapy irrespective of clinical staging, but chemotherapy can be omitted in low-grade (Grade 1) tumors. The recurrence rates of immature teratoma are 18%, 37%, and 70%, in Grade 1, 2, and 3 tumors, respectively [22], with 3-year disease-free survivals after fertility-sparing surgery being 100%, 70%, and 66% [24], respectively. While most MOGCTs have extremely high sensitivity to chemotherapy, dysgerminomas have high sensitivity to irradiation as well, and this can therefore be a potent tool for local control of such tumors. Yolk sac tumors, embryonal carcinomas, and non-gestational choriocarcinomas are rare and sometimes have mixed components of each histology type. Since tumor diameter and histological type are considered as important prognostic factors in these mixed germ cell tumors, careful pathological examination is required, with a sufficient number of histological sections [20, 24]. Large tumors of high-grade immature teratoma, or those composed of yolk sac or choriocarcinoma components in over one third of histological specimens, have a worse prognosis, while tumors with <10 cm diameter have good overall prognosis irrespective of the histological composition [25].
The initial symptoms and signs of MOGCTs include subacute pain or palpation of the pelvic mass, which are observed in 80–90% of patients [26]. Some present as acute abdominal cases due to rupture of the membranes, bleeding from tumors, or torsion. It should be noted that it is not uncommon to find that patients being treated for appendicitis or other abdominal conditions, especially those that are young or preadolescent, are occasionally diagnosed during surgery as having these tumors.
Elevation of specific tumor markers is one of the characteristics of MOGCTs, in particular AFP (alpha-fetoprotein) for yolk sac tumors, hCG (human chorionic gonadotropin) for choriocarcinomas, LDH (lactate dehydrogenase) for dysgerminomas, and SCC (squamous cell carcinoma antigen) for malignant transformation of mature cystic teratomas. However, there are a considerable number of patients without significant elevation of these markers, meaning that their diagnostic value is limited. Nevertheless, their expression can be useful to monitor residual postoperative tumor burden, as well as treatment efficacy and recurrence during follow-up.
The clinical stage of MOGCTs is determined according to the guidelines established for epithelial ovarian cancers. Extraovarian lesions of MOGCTs mainly consist of retroperitoneal lymph node metastases and peritoneal dissemination. A SEER study of 760 cases of MOGCT reported that 76% of cases were Stages I and II, while 24% were Stages III and IV [27]. The prognostic factors for MOGCT have been studied by multivariate analysis, with clinical stage and preoperative levels of tumor marker (AFP and hCG) found to be independent prognostic factors for survival in one report [28], while SEER has reported that patient age at diagnosis, clinical stage, and histological type (i.e., yolk sac tumor) were independent prognostic factors [27]. SEER also reported that patients with retroperitoneal metastasis have significantly worse 5-year survival compared to those without retroperitoneal metastasis (83% vs. 96%) and that retroperitoneal metastasis is another independent prognostic factor [29].
10.3.2 Molecular Aspects of Ovarian Germ Cell Tumors
A wide variety of molecular studies, including genome sequencing and transcriptome profiling, have characterized the biological features of MOGCTs and their potential biomarkers. The characteristic features reported for the main histological subtypes of MOGCTs are summarized in Fig. 10.2, with pure dysgerminoma and yolk sac tumors having been found to be mainly non-diploid (i.e., tetraploid, polyploid, or aneuploid), while only 8% of immature teratomas are thought to be non-diploid [30]. DNA copy number analyses have revealed that part or whole gains of chromosomal arm 12p are frequent among both MOGCTs and testicular germ cell tumors [31]. A transcriptome profiling study comparing dysgerminoma and yolk sac tumors revealed that a subset of eight WNT/β-catenin signaling components is sufficient to distinguish between the two histological subtypes [32]. Immunohistochemical analysis from the same study indicated that cytoplasmic β-catenin is expressed in all histological subtypes, but with only weak focal staining in dysgerminoma, and that β-catenin nuclear accumulation is observed only in yolk sac tumors and teratomas [32]. Other work has indicated that the IL6R (interleukin 6 receptor) and C-X-C motif chemokine 10 (CXCL10), known to be involved in cytokine signaling and immune responses, are overexpressed in dysgerminomas [30]. Upregulation of IL6R expression prevents premature entry into meiosis and maintains an immature germ cell population in the human fetal ovary [33]. On the other hand, the expression of CXCL10 and its receptor CXCR3 can lead to tumor recruitment of T-lymphocytes [34], which is in keeping with the observation of infiltration of T-lymphocytes in dysgerminoma, although the biological function and significance of this phenomenon remains unclear.
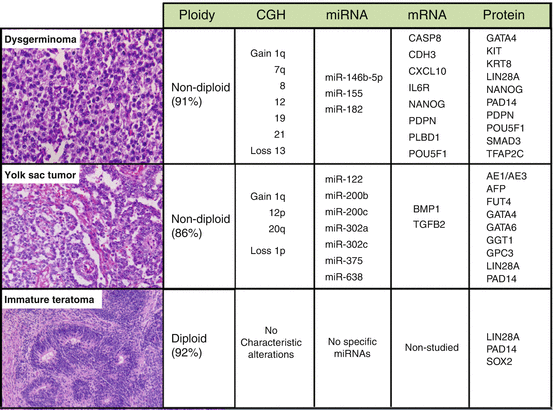

Fig. 10.2
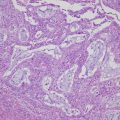
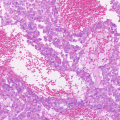
Representative molecular characteristics of the main histological subtypes of MOGCTs. Hematoxylin- and eosin-stained sections of the subtypes are illustrated to the left; dysgerminoma (growing with sheets or nests of polygonal cells with round vesicular nuclei, abundant clear cytoplasm with glycogen, and well-defined cell membranes), yolk sac tumor (commonly with reticular (left side) and endodermal sinus (right side) growth patterns) and immature teratoma (with variable amounts of immature embryonal-type tissues, mostly in the form of neuroectodermal tubules and rosettes (as shown)). The typical diploid or non-diploid, copy number alterations reported in ≥30% of the subtypes and aberrantly expressed miRNAs, mRNAs, and proteins are also listed. Adapted with alterations from Endocr Rev. 2013; 34: 339–376
The pluripotency genes, NANOG (nanog homeobox), POU5F1 (POU domain, class 5, transcription factor 1), POU5F1B (POU domain, class 5, transcription factor 1B), and PDPN (podoplanin), have also been found to be overexpressed in dysgerminoma [35] and seminoma [36]. The fact that the expression pattern for these genes is similar between dysgerminoma and seminoma indicates that common tumorigenic pathways exist for a subgroup of ovarian and testicular germ cell tumors and/or that such expression patterns represent the remnant traits of their mutual precursor, i.e., the primordial germ cell. Other groups have reported that the cell signaling genes BMP1 (bone morphogenetic protein 1) and TGFB2 (transforming growth factor-beta 2) are overexpressed in yolk sac tumors [32, 37]. The TGF-β/BMP signaling pathway regulates embryonic development, and its biological relevance is underlined by the fact that mutations in the BMP receptor Alk6b (activin receptor-like kinase 6b) impairs germ cell differentiation and initiates germ cell tumors in zebra fish [38].
Several microRNA (miRNA) expression profiling studies have identified that two miRNA clusters, namely, miR-302-367 and miR-371-373, are overexpressed in MOGCTs when compared with nonmalignant control tissues [35, 37, 39, 40]. The coordinate overexpression of these miRNAs appears to be specific for MOGCTs, with no similar findings having been reported for other malignancies or diseases to date. Gene ontology analysis has shown that the downregulated mRNA targets for miR-302-367 and miR-371-373 mediate cellular processes important in oncogenesis and malignant progression, supporting the functional significance of these miRNA clusters in the biology of MOGCTs [35]. On the other hand, the most significantly overexpressed miRNA in yolk sac tumors has been reported to be miR-375 [37, 40]. Dysregulation of miR-375 has been observed for various tumor types, including head and neck, esophageal, lung, and gastric cancers [30]. Signaling pathway analyses of miR-375-regulated genes have indicated the involvement of cell cycle regulation, focal adhesion, MAPK (mitogen-activated protein kinase), TGF-β, WNT, and VEGF (vascular endothelial growth factor) pathways [41]. In dysgerminoma, three other miRNAs have been identified as being highly expressed, namely, miR-0146b-5p, miR-155, and miR-182 [37, 40]. Although the specific functions of these miRNAs remain unclear, they are known to be overexpressed in other tumor types, including breast, lung, cervix, and colon cancers, and interactions with BRCA1 (breast cancer associated gene 1) have been reported [30].
In regard to potential biomarkers for MOGCTs, protein expression analyses have indicated that pluripotency/developmental factors and histology-specific markers may be the two most important functional categories. POU5F1 and NANOG are significantly expressed more often in dysgerminoma, for example, supporting their application as biomarkers for this subtype [42, 43]. The pluripotency factor SOX2 (sex-determining region Y-box 2), on the other hand, has been shown to be more significantly expressed in immature teratoma and to be very specific to this subtype [30, 43]. Primordial germ cells do not express SOX2 and remain capable of proliferation, and thus the absence of SOX2 expression in dysgerminoma underlines their strong resemblance to this progenitor cell type. In addition to POU5F1, PDNP has also been proposed as a diagnostic marker of dysgerminoma [30]. In contrast, the differential diagnosis of yolk sac tumor is difficult due to its complex and varied histological appearance, especially between yolk sac tumor and clear cell carcinoma of the ovary, and good markers for this tumor type are limited. Mixed tumors exhibit further complexity, with small components of yolk sac tumor growing in close proximity to other subtypes such as immature teratoma. In the past, AFP has been a famous tumor marker for yolk sac tumors [44], but the diagnostic use of AFP immunohistochemistry has low sensitivity and specificity [45]. Alternatively, the transcription factor GATA6 (GATA-binding factor 6) has been shown to be more frequently expressed in yolk sac tumors than dysgerminomas, with GATA4 (GATA-binding factor 4) being expressed in dysgerminoma, yolk sac tumors, and immature teratomas [46]. The differential expression pattern of GATA4 and GATA6 may thus be used as a marker to distinguish between yolk sac tumors and dysgerminomas.
10.3.3 Treatment Strategy of Ovarian Germ Cell Tumor
Surgery is the primary treatment of MOGCTs. Since most patients with MOGCTs are of preadolescent or reproductive ages and have unilateral tumors, fertility-sparing surgery should be considered, especially considering the fact that patients with MOGCTs are extremely sensitive to chemotherapy. Unilateral salpingo-oophorectomy of the affected side with omentectomy and peritoneal cytology are the basic procedures in operation for MOGCTs. A routine biopsy of the contralateral ovary should be avoided to preserve ovarian function, unless macroscopic findings are detected [47]. However, since dysgerminoma occasionally (in 10–15% of cases) occurs bilaterally, careful examination of the contralateral ovary is necessary [48]. Stage III and IV patients who desire fertility-sparing surgery can be permitted this option, with a focus on tumor debulking [47, 49], based on the evidence that fertility-sparing surgery does not adversely affect prognosis [26, 27, 50–52].
Intraoperative frozen section analysis is necessary irrespective of the type of surgery undertaken (fertility-sparing or otherwise). However, the diagnostic accuracy of such an analysis is of limited value, and it is recommended to avoid overtreatment during the operation. In the event that a differential diagnosis is required to distinguish the tumor from types that do not permit fertility-sparing surgery, it may be appropriate to initially perform fertility-sparing surgery without overtreatment and then reoperate if necessary after postoperative pathological examination.
When patients do not require fertility-sparing surgery, standard operative procedures for epithelial ovarian malignancies should be performed, with the addition of pelvic and para-aortic lymphadenectomy, although the prognostic impact of retroperitoneal lymphadenectomy is not proven. A recent retrospective study of 1083 patients with MOGCTs that were deemed to be at clinical Stage I at the time of surgery reported no significant difference in the 5-year survival between patients with and without retroperitoneal lymphadenectomy, including patients who were upstaged to FIGO (International Federation of Gynecology and Obstetrics) Stage IIIC after lymphadenectomy [53]. On multivariate analysis, lymphadenectomy was not an independent predictor of survival when controlling for age, histology, and race. Moreover, the presence of lymph node metastasis had no significant effect on survival [54]. Thus, neither lymphadenectomy nor lymph node metastasis was an independent predictor of survival in patients with MOGCTs confined to the ovary. This probably reflects the highly chemosensitive nature of these tumors, and retroperitoneal lymphadenectomy can thus be omitted [54].
There are issues about the selection of surgical procedures and postoperative treatments in each tumor type. It remains unresolved whether patients with Stage I (Grade III) immature teratoma, pathologically diagnosed after ovarian cystectomy for mature cystic teratoma, require the addition of adnexectomy [55]. It has been accepted, however, that there is no need for chemotherapy in patients with Stage IA dysgerminoma or Stage IA (Grade I) immature teratoma [47]. Furthermore, in patients with Stage IA dysgerminoma that undergo operation with incomplete surgical staging, chemotherapy can be delayed until there is evidence of relapse, since these tumors have been shown to respond well to chemotherapy upon recurrence [56].
The current standard chemotherapy regimen for MOGCTs is BEP (bleomycin, etoposide, and cisplatin), based on the clinical trial results for testicular germ cell tumors, as well as the excellent cure rates achieved in early-stage patients (almost 100%) and even in advanced patients (at least 75%) [57]. Despite the lack of Phase III trials, BEP is strongly recommended as standard chemotherapy regimen for MOGCTs, although special attention should be paid to guarantee the best outcomes with this approach. Firstly, drug doses should be maintained, without reckless reduction. Only in the case of pyrogenic neutropenia, or thrombocytopenia with bleeding, can a 20% decrease in etoposide be permitted [58]. Secondly, the drugs should not be substituted for alternatives. In testicular tumors, the attempt to omit bleomycin in favor of decreasing pulmonary toxicity has been shown to fail, worsening the prognosis of the patient [59]. Furthermore, a change from cisplatin to carboplatin has also been reported to adversely affect prognosis [60]. Thirdly, treatment schedule compliance is strictly important. Even with the presence of neutropenia, the next cycle of chemotherapy must commence at day 22 [61], and although the presence of severe bone marrow suppression, such as neutropenia <500 per mm3 or thrombocytopenia <105 per mm3, may permit delay of the next cycle of chemotherapy, it should only do so for a maximum of 3 days [62]. This compliance requirement is thus quite different from more common epithelial tumors of the ovary. Finally, and as mentioned above, postoperative adjuvant chemotherapy with BEP can be omitted in patients with Stage IA dysgerminoma and Stage I (Grade 1) immature teratoma [44] and is in fact recommended to be omitted in young patients (<15 years old) with immature teratoma [63, 64].
One of the critical issues in chemotherapy for MOGCTs is how many cycles should be performed, since there have been no RCTs to assess the optimal number. Based on GOG78 (in which one arm of the trial performed three cycles of BEP for early-stage MOGCTs), the NCCN (National Comprehensive Cancer Network) guidelines now recommend three cycles of BEP [55, 57]. In the BEP protocol, however, accumulative pulmonary toxicity caused by bleomycin and secondary neoplasms induced by etoposide should be a concern. The rate of occurrence of pulmonary toxicity from bleomycin is 0–2% over three cycles of BEP and is 6–18% over four or more cycles. A pulmonary function test performed during bleomycin therapy is unfortunately not a good predictor of toxicity, since it has been shown to have a relatively low sensitivity and specificity [65, 66]. Secondary neoplasms triggered by etoposide are also accumulative, and the rate of occurrence is very low (0.4%) with a total dose of less than 2000 mg/m2, but increases at doses over 2000 mg/m2 [67]. The threshold for etoposide to induce neoplasms is thus thought to be 2000 mg/m2 [68]. Prognosis of secondary leukemias caused by etoposide is poor, with most cases arising 2–3 years after initial chemotherapy, and it is thus important to monitor closely for occurrence of secondary leukemia when >2000 mg/m2 of etoposide is used [69].
There are unfortunately no RCTs comparing different regimens of chemotherapy for MOGCTs. In testicular tumors, the BEP regimen was compared with etoposide, ifosfamide, and cisplatin (VIP therapy), with no significant difference in long-term prognosis reported, although bone marrow suppression was found to be more prominent in the former [70].
Following postoperative BEP chemotherapy, the failure of ovarian function due to toxicity, as well as secondary neoplasms induced by etoposide, should be cared for in particular. Failure of ovarian function is most frequently observed when cyclophosphamide is used in treatment regimens, but BEP has shown a relatively rare rate of failure for ovarian function. Amenorrhea is frequently (62%) observed during BEP chemotherapy, but 91% of patients undergoing this regimen appear to recover menstruation [71]. In general, 80–90% of patients receiving chemotherapy for MOGCTs eventually recover menstruation following treatment [72]. It has been reported that the incidence of infertility, congenital malformation, and spontaneous abortion do not increase after MOGCT chemotherapy [37, 72–75], and there are several reports suggesting that pretreatment with GnRH (gonadotropin-releasing hormone) analogues or oral contraceptives may protect ovarian function during chemotherapy [76–78]. A randomized trial in breast cancer has reported the preservation of ovarian function by GnRH analogues during chemotherapy [79], but there is no consensus regarding the utility of such protection.
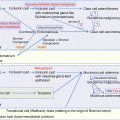
The Japan Society of Gynecologic Oncology published the guidelines for the treatment of ovarian tumors [21], and the flow chart of the treatment of MOGCTs is shown in Fig. 10.3.
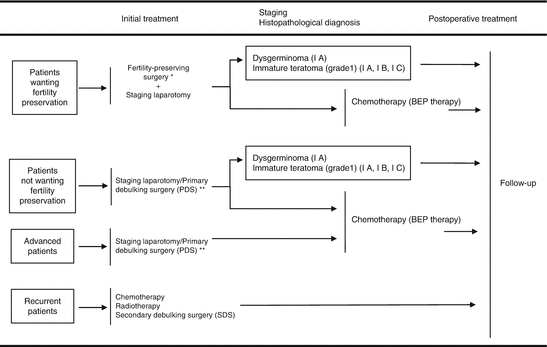

Fig. 10.3
Treatment of malignant ovarian germ cell tumors. *Fertility-preserving surgery—affected-side salpingo-oophorectomy + omentectomy + peritoneal cytology + detailed intra-abdominal examination. **Lymph node dissection (biopsy) can be omitted. Reprint with permission from ref. 21
10.4 Conclusions and Future Directions
Non-epithelial ovarian tumors are rare, and there are few RCTs for the treatment of these tumors. We therefore have limited information in regard to the most appropriate management strategy for these cancers. However, considerable efforts have been made to apply fertility-sparing surgeries for young patients, an approach that has proven to be relatively safe in early-stage tumors. Although pathological diagnosis is occasionally difficult, especially in intraoperative cases, and thus it is not always easy to judge where fertility-sparing surgery may be indicated, it is important that radical surgery be avoided in patients with difficult intraoperative diagnoses. In such cases, fertility-sparing surgery should be performed first, with radical surgery conducted subsequently only if postoperative pathological assessment indicates necessity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree