6 Strategies for Risk Reduction
For those women at increased risk of developing breast cancer, improved methods of risk and genetic assessment have occurred simultaneously with improved methods of risk reduction. Women identified as having an increased risk for development of breast cancer can be offered a variety of increased surveillance strategies or risk-reducing interventions. Chemoprevention combined with increased surveillance is one option. A second option is surgical risk reduction with prophylactic mastectomy.
The selection of women who should be counseled regarding risk-reduction strategies is dependent on reliable and thorough risk assessment (see Chapters 4 and 5). After a woman has been identified as having increased risk of developing breast cancer, a variety of options are available to her, ranging from increased surveillance to chemoprevention to surgical intervention. Multidisciplinary evaluation and management, including overall health of the woman, comorbid conditions, possible risks and benefits of each method, psychological impact of the intervention, and long-term consequences must be considered before a management strategy is implemented.
Concepts of Chemoprevention
The concept of chemoprevention was eloquently presented by Michael Sporn1 in 1975 while discussing the use of retinoids in prevention of epithelial cancers. The discussion centered on the idea that cancer prevention should be studied and implemented in the fight against cancer. The process of a normal cell becoming a cancerous cell is one that occurs over time, and if we can stop the advancement of that process we can actually prevent the cancer from occurring. Chemoprevention is the use of chemotherapies to stabilize, suppress, or reverse the mechanisms by which a precancerous lesion becomes a cancerous lesion. Breast cancer research has led the way in analyzing and implementing models of chemoprevention.
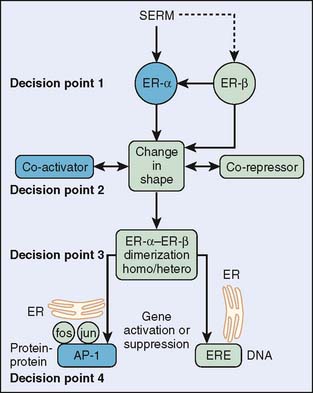
Chemoprevention for breast cancer evolved from the treatment of breast cancer. Tamoxifen, a first-generation selective estrogen receptor modulator (SERM) has been extensively used in the treatment of breast cancer. Many studies of tamoxifen use in breast cancer treatment have shown a secondary effect of reducing primary breast cancer in the contralateral breast by 40% to 50%.2–4 This finding elucidated tamoxifen as a chemopreventive agent for breast cancer and launched the first prospective randomized phase III trial of the efficacy of any chemopreventive agent—this one specifically for preventing breast cancer in high-risk women.5 Figure 6-1 shows the decision points that a SERM must pass to modulate estrogen-like actions in a target tissue.
Tamoxifen Chemoprevention Studies
In 1992, the National Adjuvant Breast and Bowel Project (NSABP) started the Breast Cancer Prevention Trial P-1 (BCPT P-1) to evaluate the potential of tamoxifen in preventing breast cancer in high-risk women.6 In this study, 13,388 women 35 years and older with an increased risk of breast cancer according to the Gail model risk factors were randomized to tamoxifen 20 mg a day or placebo for 5 years (Table 6-1). The Gail model is based on age, number of first-degree relatives with breast cancer, nulliparity or age at first live birth, number of breast biopsies, presence of atypical hyperplasia, and age at menarche.7 The three high-risk groups included women 60 years of age and older (baseline risk 1.66), women 35 to 59 years who had a predicted 5-year risk of breast cancer development of at least 1.66, and women who had a history of lobular carcinoma in situ (LCIS).8 In the final analysis (Table 6-2), the cohort’s 5-year predicted breast cancer risk ratio was an average of 2.33, whereas 70% of the women were younger than 60 years.
Table 6-1 Cohort Characteristics of the BCPT P-1 Trial
| Age (years) | |
| 35–39 | 2.6% |
| 40–49 | 36.7% |
| 50–59 | 30.8% |
| 60–69 | 24.0% |
| >70 | 6.0% |
| Ethnicity/race | |
| White | 96.4% |
| Black | 1.7% |
| Other races | 1.9% |
| Risk assessment | |
| History of lobular carcinoma in situ | 6.3% |
| History of atypical ductal hyperplasia | 9.1% |
| No first-degree relative with breast cancer | 23.8% |
| One first-degree relative with breast cancer | 56.8% |
| Two first-degree relatives with breast cancer | 16.4% |
| Three or more first-degree relatives with breast cancer | 3.0% |
| Prior surgery | |
| Women with hysterectomy | 37.1% |
Data from Fisher B, Costantino JP, Wickerham L, et al: Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90(18):1371–1388, 1998, Table 2.
Table 6-2 BCPT P-1: Percentage of Women per Risk Ratio
| Risk Ratio | Percentage of Women |
|---|---|
| ≤2.00 | 25.0 |
| 2.01–3.00 | 31.0 |
| 3.01–5.00 | 26.6 |
| ≥5.01 | 17.4 |
Data from Fisher B, Costantino JP, Wickerham L, et al: Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90(18):1371–1388, 1998, Table 2.
The results of the BCPT P-1 trial were overwhelmingly positive, and the trial was unblinded in 1998. Among women with increased risk of breast cancer, tamoxifen reduced the risk of invasive breast cancer by 49% and the risk of noninvasive breast cancer (ductal carcinoma in situ [DCIS]) by 50%.6 The amount of risk reduction varied with age and with diagnosis of LCIS and atypical ductal hyperplasia (ADH) (Table 6-3). Although women of all ages saw a benefit from tamoxifen, the greatest effect was seen in women with ADH and LCIS, with a decrease of 56% and 86%, respectively, in the incidence of breast cancer. Tamoxifen reduced the incidence of estrogen-receptor (ER) positive tumors but had no effect on ER-negative tumors. This is logical since tamoxifen targets the estrogen receptor. How then does tamoxifen affect tumors of women with mutations in BRCA1 and BRCA2? BRCA1 tumors tend to be ER negative, whereas BRCA2 tumors tend to be ER positive. Blood samples were obtained from BCPT participants at entry into the initial study. Genomic analysis of BRCA1 and BRCA2 for 288 women who developed breast cancer after entry into the BCPT was undertaken.9 Tamoxifen reduced breast cancer incidence in BRCA2 carriers by 62%, whereas it did not reduce the incidence in BRCA1 carriers who tend toward ER-negative tumors.9 In 2005, an update of the initial findings from the BCPT P-1 trial was presented. After 7 years’ follow-up, the data still support the conclusive results.8
Table 6-3 BCPT P-1: Percentage of Invasive Breast Cancer Reduced
| Total group | 49 |
| Women < 49 yr | 44 |
| Women 50–59 yr | 51 |
| Women > 60 yr | 55 |
| Women with LCIS | 56 |
| Women with ADH | 86 |
LCIS, lobular carcinoma in situ; ADH, atypical ductal hyperplasia.
Data from Fisher B, Costantino JP, Wickerham L, et al: Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90(18):1371–1388, 1998, Table 2.
Tamoxifen Risks and Complications
We cannot speak of the benefits of tamoxifen without speaking of the possible risks and complications. Women who received tamoxifen in the BCPT P-1 trial had a 2.53 greater risk of endometrial cancer. The absolute risk of developing endometrial cancer was 0.79% in the tamoxifen group compared with 0.37% in the control group. The risk was higher in women over 50 years old, who demonstrated a risk ratio of 4.01 in developing endometrial cancer while taking tamoxifen.6 Although the development of endometrial cancer has long been thought to be the major disadvantage of taking tamoxifen, all the endometrial tumors found in women in the trial were early-stage and readily treated. No deaths from endometrial cancer occurred. There was an increase in cataract development and consequent surgery for cataracts in women taking tamoxifen, with a risk ratio average of 1.35, indicating only a very slight significance.6 A 19.5% decrease in fractures was also seen in the group of women taking tamoxifen. However, this did not reach statistical significance.6
Thromboembolic events were seen at an impressively high rate and proved to be a more worrisome complication in those taking tamoxifen. Pulmonary embolus was increased by a risk ratio of 3.01.6 Pulmonary emboli were seen predominantly in women 50 years and over by a risk ratio of 3.19 compared with women 49 years and younger, who had a risk ratio of 2.03. Deep venous thrombosis was also seen more often in women who took tamoxifen compared with that seen in the control group. Again, the incidence occurred more often in women 50 years and older with a risk ratio of 1.71, compared with 1.39 in women 49 years and younger. Stroke incidence was only slightly increased in the tamoxifen group with a risk ratio of 1.59, which was not statistically significant. It is interesting that the incidence of heart disease was the same in both groups, indicating no protection from heart disease with tamoxifen.
Raloxifene Chemoprevention Studies
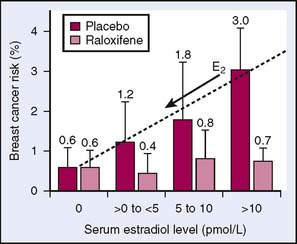
In an effort to continue to search for a drug with a high-benefit, low-risk ratio for use in the prevention of breast cancer, the NSABP initiated another study. The Study of Tamoxifen and Raloxifene (STAR) P-2 trial began on July 1, 1999, and closed on December 31, 2005.10 The study group consisted of 19,747 postmenopausal women of mean age 58.5 years with a mean risk ratio of 4.03 for developing breast cancer in 5 years (Table 6-4). The STAR trial disseminated from the Multiple Outcomes of Raloxifene Evaluation (MORE) trial and the subsequent 4-year follow-up trial, Continuing Outcomes Relevant to Evista (CORE) trial.11 These studies were undertaken to assess raloxifene in preventing fractures in postmenopausal women. A secondary outcome from the MORE and CORE trials was that raloxifene was noted to reduce the risk of breast cancer compared with tamoxifen, whereas it may have a decreased risk of endometrial cancer compared with tamoxifen.11 A subsequent study looking at the breakdown of subgroups in the MORE and CORE trials published in 2006 showed that raloxifene reduced the risk of breast cancer in postmenopausal women regardless of risk factors. However, the effect was greater in women with increased risk of developing breast cancer, varying from 33% to 89% decreases in breast cancer occurrence congruent with the estimated risk.12 This proved that the effect of raloxifene was greater in higher-risk women. Other examinations of the MORE study data have shown that the reduction of breast cancer risk seen in the use of raloxifene is dependent on the level of endogenous estrogen (Fig. 6-2).
Table 6-4 Risk Ratio According to Percentage of Women in STAR P-2 Trial
| Risk Ratio | Percentage of Women |
|---|---|
| ≤2.00 | 11.0 |
| 2.01–3.00 | 30.3 |
| 3.01–5.00 | 31.4 |
| ≥5.01 | 27.3 |
Data from Vogel VG, Costantino JP, Wickerham L, et al: Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA 295(23):2727–2926, 2006, Table 1.
The STAR P-2 trial results were published in 2006 and established that raloxifene was as effective in reducing the risk of invasive breast cancer; however, it did not reduce the risk of DCIS.10 The raloxifene group had fewer thromboembolic events and cataracts but the same risk of endometrial cancer.10 It should be noted that the rate of risk of endometrial cancer in the raloxifene group was 38% lower than in the tamoxifen group; however, it was not statistically significant. Even more importantly, the risk of thromboembolic events was decreased by 30% in the raloxifene group compared with the tamoxifen group, which was significantly different. This should be emphasized, especially considering that the group experiencing thromboembolic events more often were the women who were over 50 years and who had greater representation in the STAR study (Table 6-5). Therefore, the STAR trial gave us another useful chemopreventive agent in the fight against breast cancer, though in a different group of women. Raloxifene is an alternative for lowering the risk of breast cancer in postmenopausal women with moderate to high Gail model risk scores and in those with LCIS.10 Raloxifene has recently been approved by the Food and Drug Administration (FDA) for use in breast cancer prevention in high-risk women.
Table 6-5 Cohort Characteristics in STAR P-2 Trial
| Characteristic | Percentage of Women |
|---|---|
| Age (years) | |
| <49 | 9.0 |
| 50–59 | 49.8 |
| 60–69 | 32.4 |
| >70 | 8.7 |
| Race/ethnicity | |
| White | 93.5 |
| African American | 2.4 |
| Hispanic | 2.0 |
| Other | 2.1 |
| Risk assessment | |
| No first-degree relatives with breast cancer | 28.8 |
| One first-degree relative with breast cancer | 52.2 |
| Two first-degree relatives with breast cancer | 15.9 |
| Three or more first-degree relatives with breast cancer | 3.0 |
| History of lobular carcinoma in situ | 9.2 |
| History of atypical ductal hyperplasia | 22.8 |
| Hysterectomy | 51.4 |
Data from Vogel VG, Costantino JP, Wickerham L, et al: Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA 295(23):2727–2926, 2006, Table 1.
International Chemoprevention Studies
Another trial, International Breast Cancer Intervention Study (IBIS-1), enrolled 7154 women between 1992 and 2001.13 This was a double-blind study in which women took either tamoxifen or placebo for 5 years with a median follow-up of 8 years. The primary outcome was the occurrence of invasive breast cancer or DCIS. These women were between 35 and 70 years old and were eligible on the following criteria: at least a 2.0 relative risk of breast cancer development for ages 45 to 70, a 4.0 relative risk for ages 40 to 44, and a 10.0 relative risk for ages 35 to 39. This study was conducted in Australia, New Zealand, and the United Kingdom. Obviously, these women had a much higher cumulative risk than those in the BCPT P-1 and P-2 trials. Initial results released in 2002 indicated a risk reduction of 32%, which was not statistically significant. There was no significant increase in endometrial cancer; however, there was a significant increase in thromboembolic events.13 Of note is that nearly 50% of the thromboembolic events occurred within 3 months of major surgery or prolonged immobilization. The initial interpretation was that there was not enough evidence to support a positive benefit-to-risk ratio. In 2007, after a median follow-up of 96 months, the results subsequently confirmed that tamoxifen reduces the risk of ER-positive breast cancer in women who have a significantly increased risk for developing breast cancer.14 Note that this study indicates that the effects of tamoxifen last for at least 10 years after follow-up, whereas the major adverse effects do not continue after the 5-year treatment period.14
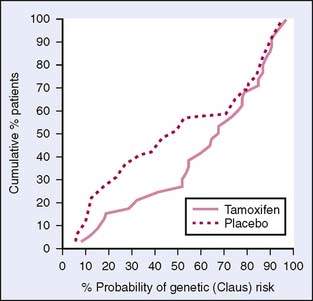
Two large European studies have also looked at tamoxifen in the prevention of breast cancer: the Royal Marsden Tamoxifen Breast Cancer Prevention Trial (Marsden) from Great Britain and the Italian Randomized Tamoxifen Prevention Trial Among Women with Hysterectomy (Italian). Early results from these trials showed minimal protective effect from tamoxifen, possibly because of the small number of women enrolled (Fig. 6-3). However, both studies have recently reported significant positive results with tamoxifen in the prevention of breast cancer in high-risk women.15,16 The Marsden trial enrolled a total number of 2471, with a median 13.2-year follow-up. The women were randomly assigned to take tamoxifen daily or placebo for 8 years. The primary outcome was the occurrence of invasive breast cancer. The results showed a 39% decrease in the incidence of breast cancer in ER-positive tumors.15 This was seen after the 8-year treatment period compared with during the treatment period. This seemed to indicate a long-term preventive effect rather than a treatment effect of tamoxifen.15 In this trial, participants were younger and had a higher relative risk that was based on their family history of breast cancer. The difference in patient characteristics and the sample size studied may account for the differences in initial findings (Table 6-6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree