PROM name
Use
What has it shown?
Comment
Authors or reference
General PROMs
Medical outcomes study short-form health survey (SF-36 and SF-12)
Studies of populations with multiple diseases
Hard to show differences in global scores
Has been supplanted by newer disease-specific modules
Ware et al. [19]
EORTC QLQ-C30
Assesses quality of life in cancer patients and offers optional disease and context-specific modules with additional symptoms
Only moderate agreement with FACT [20]
Has been used in more than 3,000 studies; current version is 3.0
Aaronson et al. [3]
Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE)
An attempt by NCI to develop a PROM extension to CTCAE
Not yet used widely—Pubmed search of PRO-CTCAE yields 4 articles versus 1,343 for EORTC QLQ-C30
Used in multiple clinical trials
Basch et al. [21]
Euroquol-5 (EQ-5D)
Provides a single summary index by applying weights based on the valuation of EQ-5D health states from general population samples
Measures cost utility, or the change in a unit of “utility” related to QOL per additional cost; often relied upon by European regulatory authorities in evaluating oncology therapies
Used by many pharmaceutical companies, recommended for use in cost-effectiveness analyses by the Washington Panel on Cost Effectiveness in Health and Medicine
Rabin et al. [22]
Functional Assessment of Cancer Therapy (FACT)
Assesses quality of life in cancer patients and offers optional context-specific modules with additional symptoms
FACT-Lung scores predict survival in non-small cell lung cancer treatment studies [23]
Widely used in the United States
Cella et al. [24]
PROMIS
Measures selected symptoms and HRQOL
Patients with chronic disease have poorer HRQOL than those without a diagnosis N = 21,113 [25]
Cella et al. [26]
Domain-specific PROMs
Palliative Care Outcome Scale (POS)
A PROM for patients with advanced cancer that assesses the key goals of palliative care
Used in measuring quality of care in patients who are dying
Hearn et al. [27]
EORTC QLQ-30 Pancreas Module (QLQ-PAN26)
Disease-specific QLQ-C30
Commonly used to measure quality of life in patients with pancreatic cancer
Fitzsimmons et al. [28]
FACT-Prostate Cancer (FACT-P)
A 12-item prostate cancer subscale for FACT
Used to assess effectiveness of abiraterone [29]
Esper et al. [30]
Lung cancer symptom scale
8-min survey with nine patient and six observer items
Used to assess disease-specific burden such as fatigue, cough, dyspnea. QOL was not worse during pemetrexed maintenance versus placebo [31]
Being used by pharmaceutical companies to document symptom burden and the need to reduce it [32]
Hollen et al. [33]
Expanded Prostate Cancer Index Composite (EPIC-26)
Evaluates patient function and bother (urinary, sexual, bowel, and hormonal domains) after prostate cancer treatment
Gaining in popularity and used to develop symptom severity groups for assessing recovery after surgery
Wei et al. [34]
Coverage of symptoms by the general PROs falls into two categories (Table 22.2). The FACT, PRO-CTCAE, and QLQ-C30 measure most symptoms used in general HRQOL studies, while PROMISE and EQ-5D measure only a small subset of those symptoms but complement them with additional assessments such as physical function and satisfaction with social roles and activities.
Table 22.2
Symptom coverage of commonly used patient-reported outcome metrics (PROMs)
Symptom | FACT | PROMIS | PRO-CTCAE | QLQ-C30 | EQ-5D |
|---|---|---|---|---|---|
Anorexia | X | X | X | ||
Anxiety | X | X | X | X | X |
Constipation | X | X | X | ||
Depression | X | X | X | X | X |
Diarrhea | X | X | X | ||
Dyspnea | X | X | X | ||
Fatigue | X | X | X | X | X |
Insomnia | X | X | X | X | |
Nausea | X | X | X | ||
Pain | X | X | X | X | X |
Neuropathy | X | X | X | ||
Vomiting | X | X | X |
There have been difficulties in using PROs and PROMs on both the scientific and administrative fronts. Scientifically, many trialists wanted to measure an average QOL score at the beginning of the trial on all the patients, then compare this to a score 2 months later. At first, this was done by taking the average of all the patients’ scores at time zero and at 2 months, which inevitably showed no difference. This would be like taking the average of the whole group of tumor responses, rather than the changes in each individual’s cancer. Better reporting and better computer entry have fixed this issue.
Another scientific issue has been defining the minimal amount of change in QOL that is clinically significant. While even small benefits in overall and progression-free survival have been used to justify drug use, the amount of “improved quality of life” has less universal acceptance. An early conference [35] agreed on common metrics: first, ask the patients what a clinically important change score would be, for example, 20 % change in pain. Second, ascertain what percentage of patients achieved that level of change. Such easily understandable and reportable metrics have allowed comparison across studies.
An additional issue has been whether to use patient scores, clinician scores, or both; and if both, in what order, and whose scores take precedence. Current data show that patient symptom scores, in addition to clinician scores, more accurately predict survival of patients than either set of scores alone [36]. In a study of 161 lung cancer patients followed for 12 months, patient-reported symptoms and QOL with the Euro-QOL 5 were complementary to clinician-reported toxicity scores using the NCI CTAEC. The clinician-reported scores were more predictive of emergency room visits and death than patient-reported scores for fatigue (p < 0.001), nausea (p = 0.01), constipation (p = 0.038), and Karnofsky Performance Status (p < 0.001), but the patient scores more accurately portrayed day-to-day function [37]. (This study also suggests that when these patient-reported symptoms rise to the level of clinical recognition they are serious and should be addressed.)
The main administrative hurdle to use of PROs and PROMs in clinical trials arises because most trials are done by pharmaceutical companies to satisfy registration (new drug approval) requirements. To date there has been only one drug approved for cancer based on a purely palliative endpoint: mitoxantrone. All others have been approved based on changes in survival, some as small as 2 weeks (median change 0.33 months) for the addition of erlotinib to gemcitabine in advanced pancreas cancer [38]. Most trials report the PRO scores separately from the main study results as a complement; of 77 recent trials in castrate-resistant metastatic prostate cancer only 18 % had PRO or tolerability companion studies [39]. For instance, sipuleucel-T improved survival by about 4 months [40], and had predictable declines in QOL during treatment due to fatigue, but by week 26 and thereafter QOL was identical between treatment and placebo groups [41]. Until the FDA changes its rules, PROs and PROMs will be an “add on” to the trials rather than the main endpoint [42].
A second administrative hurdle is related to the electronic medical record, which should be able to integrate PROs with little or no dif-ficulty, and could then be used for comparative effectiveness research. For instance, does cabazitaxel when given to patients with co-morbidities such as congestive heart failure have the same favorable effects as in clinical trial subjects [43]? The main questions would be what to measure and record for universal access, within the bounds of what is possible and not too expensive. A recent review [44] explored the “architecture” of such decision-making and proposed some rationality before we attempt to add patient portals with multiple instruments, none of which are standardized, to the mix. This will require adoption of one or several instruments capable of cross-talking and interpretation, with heightened privacy requirements and more expense, when the EMR vendors are trying to carve out market share. Such improvements are possible but will likely require major governmental intervention similar to “meaningful use” requirements.
Measuring Pain in CRPC
Pain is measured by nearly all PROMs, is associated with decreased survival in CRPC patients [45], can be debilitating and impair QOL [46], and, as was illustrated in the approval of mitoxantrone by the FDA mentioned earlier, can be an important endpoint defining treatment benefit in clinical trials. Measuring pain as a PRO is challenging. The FDA has raised its standards for measuring pain, describing how it determines whether PRO measures are adequate for use in clinical trials to support labeling claims [47]. Despite this published guidance, in the 17 years since approving mitoxantrone for the treatment of pain, the FDA has not allowed the inclusion of a pain endpoint in a prostate cancer drug label—despite multiple pain endpoints in FDA drug applications during this time [48]. Pivotal trials for both docetaxel and abiraterone acetate measured pain [7], but FDA concern about pain measurement techniques in those trials caused there to be no mention of pain reduction in the drug labels [49].
Without a viable consensus on a method for measuring pain, investigators develop pain questionnaires on a case-by-case basis with little regard for inter-trial comparability. However, a five-site clinical trial opened late in 2013, with approval from the FDA, to study a simple pain measurement PRO: “pain at its worst in the last 24 hours” [50]. This new measure is promising both because the trial design team includes the developer of the most commonly used pain questionnaire in oncology, Brief Pain Inventory (BPI), and because the trial has the active encouragement of the FDA. Results are anticipated in 2016.
Other PROs and PROMs That Clinicians May Encounter in Treating CRPC Patients
Although pain dominates discussions of PROs in CRPC patients, there is little agreement on other PROs most relevant to CRPC patients and treatment. Scher et al. suggest “other important PROs for consideration in trials and practice include anorexia (decreased appetite), anxiety, constipation, diarrhea, sleep disturbance, mucositis, nausea, pain, peripheral sensory neuropathy, rash, vomiting, urinary symptoms, global health-related QOL, and interference of symptoms with usual activities” [51]. In attempting to develop a conceptual framework of PROs for CRPC, Eton et al. developed an initial list of CRPC-relevant PROs through a literature review and interviews with patients and clinicians and then compared the resulting list with archived PRO data from a randomized phase III clinical trial of mitoxantrone with prednisone versus prednisone alone. The archived data included both the Prostate Cancer-Quality of Life Instrument (PROSQOLI) and the QOLM-P14. The result is the proposed conceptual framework for CRPC-relevant PROs shown in Fig. 22.1.
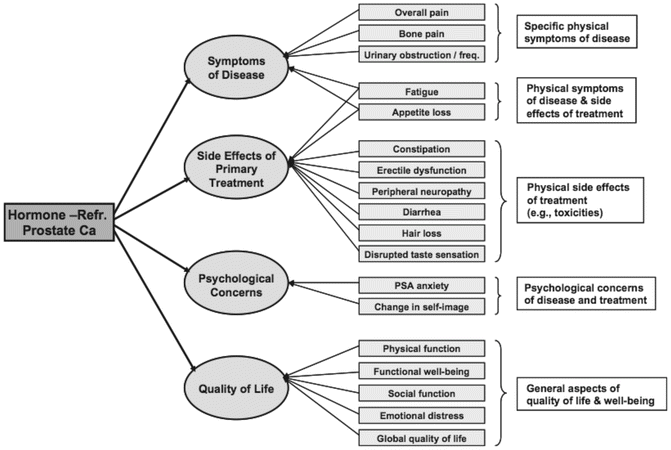

Fig. 22.1
Conceptual Framework for PROs in Castration Resistant Prostate Cancer used with permission from Eton et al. [52] This figure was published in the Journal of the International Society for Pharmacoeconomics and Outcomes Research, 13(5), Eton DT, Shevrin DH, Beaumont J, Victorson D, Cella D, Constructing a conceptual framework of patient reported outcomes for metastatic hormone-refractory prostate cancer, p. 613–23, Copyright Elsevier
Eton et al. also provided a summary of PROs observed at baseline and measured on treatment in clinical trials targeting CRPC patients (Table 22.3). Pain, prostate cancer-specific concerns, and general QOL were most frequent.
Table 22.3
Patient-reported outcomes showing change in HRPC clinical studies (number of times identified per treatment arm)
Concern or domain | Count |
|---|---|
Pain | 10 |
Prostate cancer-specific concerns (PCS)a | 9 |
Global QOL | 8 |
Emotional function | 5 |
General QOL (total scores of measure—i.e., FACT-P)b | 5 |
Fatigue | 4 |
Physical function | 4 |
Nausea/vomiting | 3 |
Analgesic use | 2 |
Functional well-being/role function | 2 |
Depression | 1 |
Appetite | 1 |
Earlier, Yount et al. extracted the most important PROs to monitor for advanced prostate cancer from a commonly used multi-dimensional prostate-cancer-specific QOL survey, the Functional Assessment of Cancer Therapy-Prostate (FACT-P). Forty-four expert clinicians, each of whom had treated at least 100 patients with advanced prostate cancer over at least 3 years, selected a list of no more than 5 of the “most important symptoms or concerns to monitor when assessing the value of treatment for advanced prostate cancer.” The researchers validated the experts’ selection in a phase III randomized clinical trial of atrasentan in 288 men with CRPC [53]. Table 22.4 shows the ranked list of PROs that were endorsed by at least 17 % of the experts.
Table 22.4
Most important symptoms in CRPC, selected by clinicians
Symptoms/concerns | % Endorsed (“top 5”) |
|---|---|
Pain | 68 |
Fatigue (lack of energy) | 64 |
Pain limits performance | 43 |
Difficulty urinating | 32 |
Worry condition will get worse | 27 |
Bone pain | 25 |
Weight loss | 18 |
Urinating problems limit activity | 18 |
In the following sections we review the character of each of these PROs and the treatments that have proven to be most effective.
Common Pain Syndromes and Treatment Strategies for Pain in CRPC Patients
Bone metastases occur in 80 % of men with advanced prostate cancer [54]. The most common site for prostate cancer metastases is bone, especially in the spine, pelvis, humeri and femurs, and less commonly in other areas such as the clivus in the skull. Bone metastases frequently cause substantial pain, pathologic fractures, and can spread and develop into spinal cord compression [55].
Effective control of pain as well as reduction in risk of fracture and cord compression in CRPC depends on prompt recognition of the specific pain syndrome the patient is experiencing. Common pain syndromes in CRPC are shown in Table 22.5.
Pain syndrome | Initial management | Other therapeutic alternatives |
|---|---|---|
Localized (focal) bone pain | Pharmacologic pain management (narcotics) Localized radiotherapy (special attention to weight-bearing areas, lytic metastasis, and extremities) | Surgical stabilization of pathologic fractures or extensive bone erosions Epidural metastasis and cord compression should be evaluated in patients with focal back pain Radiopharmaceuticals should be considered if local radiation therapy fails
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|