Stereotactic Body Radiation Therapy
William G. Rule
Sheena Jain
Thomas P. Boike
Introduction
Stereotactic body radiation therapy (SBRT) involves the precise delivery of ablative doses of focused radiation utilizing unique beam arrangements, specialized immobilization, and image guidance equipment. The general principles that guide the optimal delivery of quality SBRT have their roots in cranial stereotactic radiosurgery. Originally described by Lars Leksell in 1951, cranial stereotactic radiosurgery was a novel method of noninvasively treating intracranial pathology (1). In 1967, Leksell and his colleagues developed the Gamma Knife. The Gamma Knife relied upon a stereotactic head-frame coordinate system and multiple cobalt-60 sources to precisely deliver an ablative dose of highly conformal radiation to the target while simultaneously sparing surrounding normal tissues through steep dose gradients. Since that time, numerous investigators have efficiently exploited and confirmed the clinical value of cranial stereotactic radiosurgery (2,3,4,5,6,7). Although there was significant interest in extending the cranial radiosurgical paradigm to extracranial targets, it was not until technical developments in the linear accelerator, imaging, and immobilization arenas came to fruition that SBRT became feasible. In 1994, Lax and Blomgren, from the Karolinska Institute in Sweden, became the first to publish a system of extracranial stereotactic radiotherapy (8). Since that time, SBRT has grown by leaps and bounds and has found applications in multiple disease sites and clinical scenarios. This chapter will guide the reader through the conceptual and practical framework that surrounds the delivery of SBRT. In addition, published clinical experience in various disease subsites will be presented and summarized.
Rationale and Goals
The basic radiobiological rationale underlying SBRT is similar to that underlying cranial stereotactic radiosurgery and radiotherapy: A small number, typically 1–5, of high dose fractions delivered over a brief period of time results in a more potent biological effect than what would be expected for the same total dose delivered via conventional fractionation (9). In addition, there is evidence that the high doses utilized in typical SBRT regimens appear to take advantage of microvascular endothelial cell apoptosis to effect tumor cell kill (10,11).
While the argument for the noninvasive and potentially curative nature of SBRT delivery in, for example, a medically inoperable early stage non–small-cell lung cancer patient is relatively easy to make, the delivery of SBRT to sites of metastatic disease requires the examination of several oncologic theories/concepts:
Theory of Oligometastases—This theory puts forward the concept that there may be a group of patients with metastatic disease that exists in a discrete number of known sites. Patients with this limited burden of metastatic disease have the potential to be cured if their limited metastatic deposits are eradicated (12,13,14,15,16,17,18,19,20,21).
Patterns of Failure/Consolidation Concept—Systemic therapy alone may not be sufficient to completely eradicate all clonagens in sites of gross disease. The addition of a potent local consolidative therapy may address the regions of disease that systemic therapy is least likely to successfully address (19,20,21,22,23,24).
The Norton–Simon Hypothesis—Originally utilized in the chemotherapeutic arena, this hypothesis states that tumor response to chemotherapy is proportional to tumor growth rate. In simple terms, the proportion of clonagens killed by a particular chemotherapeutic regimen is greater when the number of clonagens present is low. A potent local therapy such as SBRT, when directed at selected sites of gross disease, may reduce the systemic disease burden and may result in chemosensitization of the remaining clonagens (19,20,21,25,26,27).
Regardless of the specific rationale utilized, the delivery of ablative doses of radiation mandates a reduction in the normal tissue volume receiving high-dose exposure. This can only be accomplished with meticulous patient immobilization, respiratory motion management, specialized dosimetric/planning considerations, and image guidance for accurate target localization.
Simulation
SBRT simulations consist primarily of three intertwined components. They are: patient immobilization, tumor motion assessment/control, and image acquisition for planning. This process should be directed by the treating radiation oncologist with a physicist either at the simulation or available for consultation. These three aspects set the stage for a safe and successful treatment.
Prior SBRT experiences and trials utilized stereotactic frame-based treatment delivery for daily realignment and patient immobilization. External fiducials within the frame were used to realign patients for treatment. This has been found to be both effective and accurate for a variety of disease sites (8). From that initial experience, SBRT has evolved from an external frame-based approach to one that relies heavily on image guidance. This has made the need for a traditional stereotactic frame obsolete. However, several aspects of the frame should be carried forward for patient immobilization. Patient positioning needs to be comfortable, secure, and reproducible. Typically, patient-specific immobilization consists of a custom-formed pillow that provides ample surface contact with the patient to assure a secure position that will remain consistent during the SBRT treatment. For reproducibility, the patient’s arms are typically placed up and out of any fields. This arm position is the major source of discomfort during treatment and the custom-formed pillow must support the arms. Vendors now offer several variations of the body frame with stereotactic localizers and most allow for abdominal compression (30,31,32,33).
Frameless approaches that use continuous monitoring of the patient are currently available. These approaches can work well provided that the system can detect and correct for changes in patient positioning. The most widely used and available system is the CyberKnife Image-Guided Radiosurgery System (Accuray, Sunnyvale, CA). This system using a linear accelerator mounted on a robotic arm to deliver treatment while periodic orthogonal kV images are taken to monitor motion. Adjustments for patient positioning and even fiducial/tumor tracking can be made with these images. The system has been shown to have an accuracy of 1.1 ± 0.3 mm for a planning CT slice thickness of 1.25 mm (34). These complex treatments have been very time consuming (35) but an update to the hardware has increased the dose rate and added a resizable collimator that should reduce treatment times.
It is recommended that motion be limited to reduce the target size and thus reduce normal tissue exposed to a high radiation dose. There are several strategies for motion control such as abdominal compression, breath-hold, gating, and tracking which have been described in detail in the Report of AAPM Task Group 76 (36). Abdominal compression is easy to adopt when starting a SBRT program, is tolerated well by patients, and allows for fast treatment delivery. Motion is first assessed using fluoroscopy to visualize the tumor or a surrogate such as the diaphragm or fiducials. Compression is applied with a paddle placed on the patient’s upper abdomen to limit the excursion of the diaphragm and encourage regular shallower breaths. Patients are coached to expand their chest wall and avoid putting pressure on the compression device. Together, this results in decreased tumor motion for lung and liver targets (37). Pressure is then increased until motion is reduced to less than 1 cm.
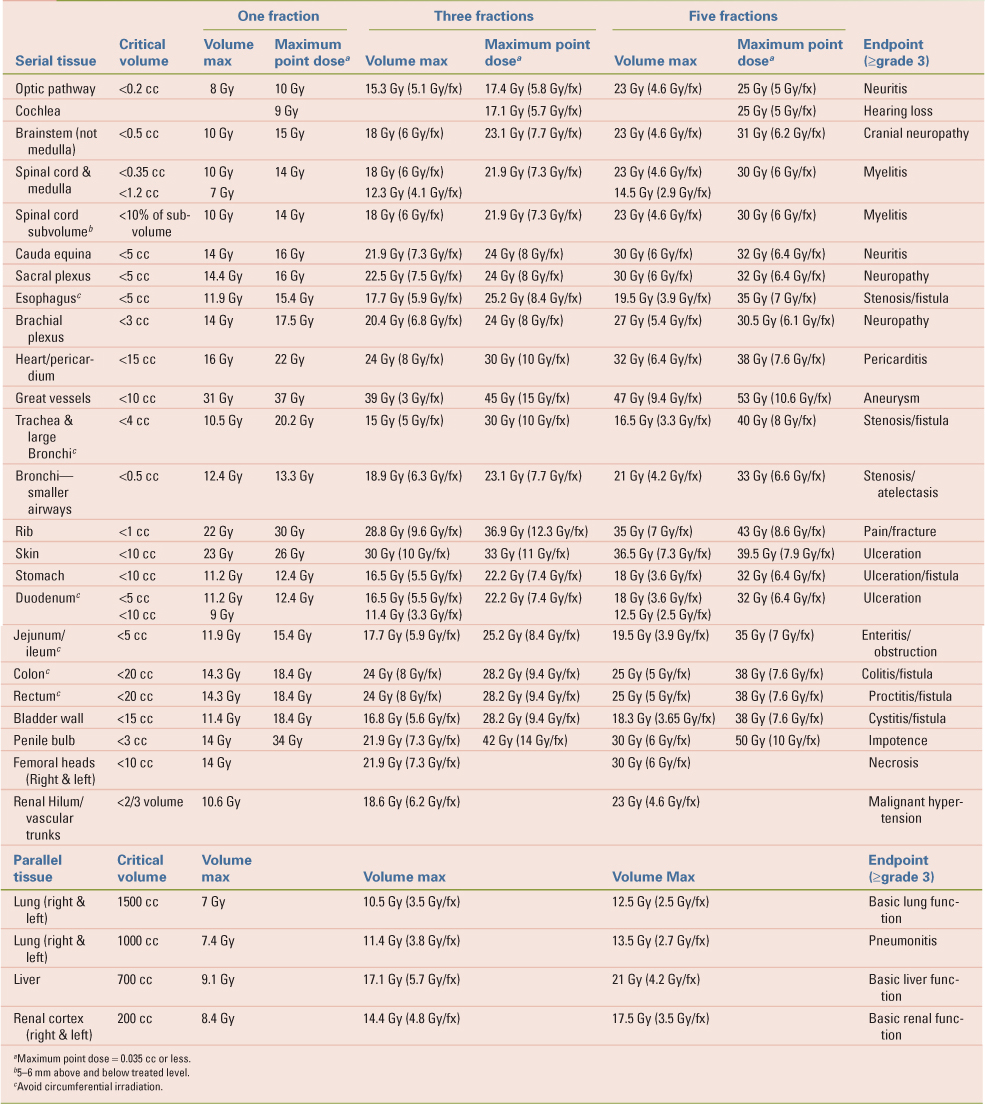
Passive and active breath-hold techniques for SBRT have been described with various devices (38,39,40). Typically, these techniques measure the patient’s respiratory cycle and have the patient hold their breath at a moderate deep-inspiration position. These have been shown to be accurate within an average of 2 to 3 mm (38). Patients must be trained for the best results, often at separate visits, and not every patient may be able to tolerate this approach. The length of each breath hold is, on average, 12–16 seconds. This strategy adds time when the treatment beam will be off, thus lengthening the treatment time of an already long radiation treatment visit. This approach may be best applied to patients with liver tumors, other abdominal targets, or metastatic disease to the lung as patients with lung cancer may have difficulty holding their breath due to compromised lung function (Fig. 16.1).
Respiratory gating is another strategy for dealing with diaphragm-related motion. A patient’s respiratory cycle is tracked using infra-red cameras and reflectors placed on the patient (41). Alternatively this could be done with stereoscopic video cameras. During treatment planning, a gating window is designed to treat in one or several of the respiratory phases. For treatment delivery, the radiation beam is turned on only when the patient is in the selected part of the respiratory phase. Respiratory gating increases treatment delivery time compared with nongating approaches with published duty cycles (ratio of beam on
time to total beam delivery time) of 30–50% (42,43). The correlation of internal targets to the external surface markers is an area of concern, especially with lung targets (44). An increased dose rate may help shorten treatment delivery. The ideal patient for gated delivery has large amplitude of respiratory motion and a small lung or liver target. It is an area of controversy if the added complexity and time of the treatment outweigh the benefit in other patients.
time to total beam delivery time) of 30–50% (42,43). The correlation of internal targets to the external surface markers is an area of concern, especially with lung targets (44). An increased dose rate may help shorten treatment delivery. The ideal patient for gated delivery has large amplitude of respiratory motion and a small lung or liver target. It is an area of controversy if the added complexity and time of the treatment outweigh the benefit in other patients.
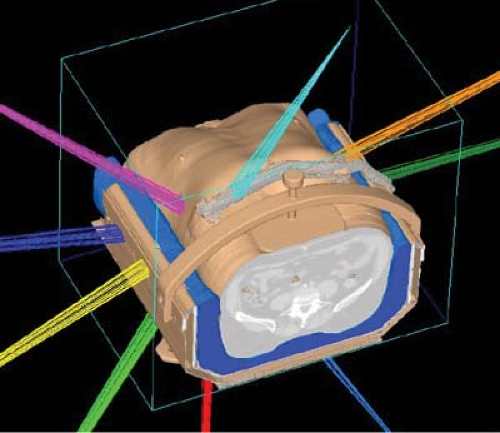 Figure 16.1. Reconstructed simulation CT of a liver patient showing the use of non-coplanar beams and abdominal compression. |
SBRT necessitates accurate image acquisition for target definition, identification of organs at risk, and treatment planning. This is traditionally accomplished with CT-based images. All organs at risk need to be covered within the scanned volume as well as any potential beam paths. Since noncoplanar beam arrangements are often utilized the volume scanned for treatment planning is often large, extending for 25 cm or greater above and below the target. Slice thickness of <3 mm is recommended for all clinical cases of SBRT. Imaging of moving targets is described in detail in AAPM Task Group 76. This includes slow CT, breath-hold techniques, gated approaches, and 4D CT (36). 4D CT can be reconstructed into maximum, minimum, and average intensity projections. Each of these has their individual strengths and limitations (45,46).
Fused images may aid in identification of targets or organs at risk. For example, prostate and liver targets may be best outlined on MRI. Regarding the spinal cord, it is best seen on T2 MRI images. When images are fused, it is important to make sure that they are acquired in the treatment position with all of the appropriate treatment immobilization devices in place. 18F-fluorodeoxyglucose (18FDG) positron emission tomography (PET) is difficult to use for SBRT but may be helpful in lung and liver targets. However, this should be used with caution as no standardized uptake value (SUV) threshold is standard for tumor definition. In addition, motion artifacts can be created due to the separate acquisition of the CT and PET images. Respiratory-correlated PET-CT may help to minimize artifacts when PET is needed as a fusion dataset for planning (47).
Table 16.1 SBRT Couch and Gantry Angles for Right and Left-Sided Lung Lesions | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Dosimetry
As previously mentioned, minimizing the volume of normal tissue exposed to high dose regions is of primary importance in SBRT. As such, the GTV is typically assumed to be equal to the CTV (48,49). In general, 0.5 cm radial margins and 0.5–1.0 cm superior-inferior margins are added to yield the PTV. These margins may be judiciously modified depending on an individual patient’s target motion characteristics as well as individual centers’ confidence in their setups.
The two main dosimetric goals in the execution of quality SBRT are a high degree of conformality and dose falloff that is rapid and isotropic (8,48,49,50,51,52). The primary means of achieving a high degree of conformality and rapid and isotropic falloff is the utilization of a relatively large number of nonoverlapping noncoplanar beams (52). Maximal separation of beam intersection (in three dimensions) and avoidance of critical structures must be balanced against the reality of the mechanical constraints and restrictions imposed by modern linear accelerator heads and couches (53). Table 16.1 lists the standard set of beams utilized for right- and left-sided lung lesions at UTSW. Beam weighting should, in general, seek to evenly distribute entrance doses in order to achieve isotropic dose falloff and to limit the possibility of skin toxicity (51,54). In lesions in which isotropic falloff completely surrounding the target may not be ideal (e.g., spinal lesions), beams may be selected to maximize falloff in certain directions in order to minimize
critical structure exposure. Such situations may also benefit from intensity-modulated planning techniques (51,55,56).
critical structure exposure. Such situations may also benefit from intensity-modulated planning techniques (51,55,56).
Choice of beam energy as well as beam-shaping parameters may also affect dose falloff as SBRT targets are often relatively small. Beam penumbra increases with increasing beam energy (secondary to lateral electron disequilibrium) and this effect becomes more prominent with targets residing within the lungs. Thus, a compromise must be reached between the desire for beam penetration and the desire to control penumbra effects. On most modern linear accelerators 6 MV is the typical photon energy of choice to treat lung targets. Higher energy beams are reserved for targets in other locations (liver, spine, etc.) or in a small number of the total beams for lung targets that must penetrate a significant amount of soft tissue. With regard to beam-shaping parameters, the vast majority of current SBRT treatment delivery is accomplished with multileaf collimators (MLCs). While very fine MLC leaf width (≤3 mm) can theoretically improve dose conformality, 5 mm leaf width appears to be adequate for most SBRT targets (21,56,57,58).
Another important consideration in planning is the utilization of tissue heterogeneity correction (especially in the setting of lung targets). In a dosimetric analysis of SBRT lung plans with and without heterogeneity corrections from the Radiation Therapy Oncology Group (RTOG) 0236 study, dose modifications were found to be necessary for future protocols utilizing heterogeneity corrections (59). Most modern treatment planning systems utilize algorithms that appropriately account for 3D scatter integration though for specific recommendations, the reader is encouraged to refer to AAPM Task Group 65 (21,60).
SBRT plans must be evaluated carefully, with close attention devoted to target coverage, conformality, normal tissue constraints, as well as the specific locations and falloff characteristics of high and intermediate dose regions. As in intracranial SRS applications, a heterogeneous dose distribution within the target volume is considered acceptable for SBRT (and potentially advantageous as the central portions of tumors often harbor hypoxic cells) (61). In SBRT, this target heterogeneity is achieved by placing the beam block/aperture margins from all beams in close adherence to the outline of the PTV. The resulting prescription lines that conformally cover the PTV are in the 60–90% range (typically ∼80%). In general, the prescription isodose line is chosen so that 95% of the PTV is conformally covered by that line and that at least 99% of the PTV is covered by a minimum of 90% of the prescription dose. The plan is normalized so that 100% corresponds to the maximum dose delivered to the patient, with that point ideally located in the center of the PTV (51,59). Conformality can be evaluated by several methods, the simplest of which is by examining the ratio of the prescription isodose volume to the volume of the PTV (59,62).
Table 16.2 lists the normal tissue tolerance doses for single-fraction, three-fraction, and five-fraction SBRT delivery utilized at UTSW. The reader is advised to be cognizant of the fact that normal tissue tolerances for SBRT delivery have been developed relatively recently and are constantly evolving as the peer-reviewed SBRT literature base expands. With regard to the location of high dose regions, every effort should be made to constrain doses greater than 105% of the prescription dose to within the volume of the PTV. Thus, the cumulative volume of tissue outside of the PTV that receives greater than 105% of the prescription dose should equal no more than 15% of the PTV volume (51,59). Intermediate/low-dose spillage is evaluated by examining the maximum dose, 2 cm away from the surface of the PTV (D2 cm) as well as the ratio of the 50% prescription isodose volume to the volume of the PTV (R50%). Tables listing D2 cm and R50% values can be found on the RTOG website (www.rtog.org).
Quality Assurance
While SBRT may use techniques similar to 3D conformal radiotherapy and IMRT, it is very unique due to the delivery of high doses to small volumes and the amount of technology utilized throughout the patient’s treatment course. This physicist plays an important role in quality assurance and should be intimately involved from simulation to treatment delivery with each patient. Accuracy of imaging/fusion, motion assessment/control, treatment planning, patient repositioning/monitoring, and treatment delivery must be confirmed with phantom measurements. This should be done with the commissioning of new equipment to establish a baseline. Ongoing treatment-specific quality assurance should be aimed at reducing systematic errors. Performance of the equipment in the hands of your department should be verified and will aid in developing PTV expansions needed to cover targets reliably. The AAPM Task Group 101 outlines best practice guidelines in SBRT. In addition, suggestions for IGRT/SBRT annual, monthly, and daily QA and tolerances have been published (21) (Fig. 16.2).
While phantom measurements for commissioning and establishing machine tolerances are important, they represent a best-case scenario. Patient-specific departmental QA procedures that ensure consistency for imaging, contouring, normal tissue dose tolerances, target coverage, motion control, treatment verification, and documentation are necessary. Prior to treatment, verification of beam configurations and treatment plans for a given patient should be verified. This can be accomplished with a machine dry run to make sure all angles that are planned are achievable. Also, an absolute point dose and fluence measurement should be made with a small ion chamber and film due to the small fields and steep dose distributions of SBRT. Having well-trained physicist, therapists, and radiation oncologist is necessary because in the end, patient safety and accurate treatment is the responsibility of the entire team.
Table 16.2 Normal Tissue Dose Constraints for SBRT Delivery | |
|---|---|
|
Early Stage Lung Cancer
With an estimated 222,520 new lung cancer cases diagnosed in 2010 and 157,300 deaths, lung cancer remains the leading cause of cancer mortality for men and women (63). Of the new diagnoses, approximately 80% will have NSCLC, and roughly 15–20% of these with have early stage disease. Standard therapy for early stage lung cancer is surgical resection with either a lobectomy or pneumonectomy. Five-year overall survival from surgical resection ranges from 60% to 70% (64,65). However, some patients are not considered candidates for resection given multiple comorbidities, poor cardiac function, or limited pulmonary reserve. In these cases, less extensive surgery with wedge or segmental resections is considered. Limited resections have been associated with higher death rates and increased locoregional recurrence, when compared to lobectomy (66). Primary conventionally fractionated radiotherapy has been utilized in these patients, with 5-year overall survival rates of 10–30% (67,68).
SBRT has been employed in early stage lung cancer with a number of fractionation schemes and doses with evidence of local control >85% (see Table 16.3). A phase I study performed by Timmerman et al. studied dose escalation in medically inoperable, early stage NSCLC. Dose escalation was started at 24 Gy in 3 fractions and was escalated to 2 Gy per fraction up to a total dose of 66 Gy. Dose was calculated without correction for tissue inhomogeneity. The maximum tolerated dose was found to be 22 Gy × 3 in large T2 tumors (5–7 cm), but was not reached for smaller tumors (32,69). A follow-up phase II study evaluating 70 patients with stage I NSCLC treating patients to 60–66 Gy in three fractions illustrated a 3-year local control of 88% and overall survival of 43%. Increased toxicity was seen in patients with “central” lung tumors, defined as tumors within 2 cm of the proximal bronchial tree (70,71).
A multi-institutional, phase II trial, RTOG 0236 assessed SBRT for peripheral lung tumors. Dose was calculated without correction for tissue inhomogeneity at 60 Gy in three fractions. Results illustrated a 3-year primary tumor local control rate of 98%, tumor plus lobe local control of 91%, and overall survival of 56%. There
were two grade 4 adverse events and there was no treatment-related mortality (72).
were two grade 4 adverse events and there was no treatment-related mortality (72).
Table 16.3 Prospective Trials in Lung SBRT | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Alternative fractionation schemes have been studied in the United States. Chang et al. at MD Anderson evaluated 27 patients with 50 Gy in four fractions. With a median follow-up of 17 months, local control was 100% with 8% developing mediastinal lymph node metastases and 15% developing distant metastases. One patient developed a brachial plexopathy and three patients (11.1%) developed grade 2 to 3 dermatitis and chest wall pain (73).
In Japan, there is extensive experience with utilizing SBRT in early stage lung cancers. In the largest retrospective series from Japan, published by Onishi et al., 257 patients were treated with SBRT from 14 centers. Doses were delivered at 3–12 Gy in 1–22 fractions and with a 38 month follow-up, local control was 86%. Three- and 5-year overall survival were 57% and 47%, respectively. Patients receiving a BED >100 Gy were found to have higher local control and survival rates (74). A phase I/II study performed by Nagata et al. evaluated 45 patients treated with 12 Gy × 4. No grade 3 toxicities were noted and 3-year overall survival was 83% for stage IA and 72% for stage IB (75).
There are multiple multi-institutional RTOG trials that are further studying the role of SBRT in the treatment of early stage lung cancers. RTOG 0618 is a phase II trial, recently closed to accrual, that is evaluating the role of SBRT in operable stage I/II NSCLC treated to 60 Gy in three fractions. RTOG 0813 is a phase I/II trial evaluating SBRT for early stage, centrally located, NSCLC in medically inoperable patients. In addition, RTOG 0915 is a phase II study comparing 34 Gy in one fraction versus 48 Gy in four fractions for inoperable, peripherally located tumors. The least toxic of these two arms will be compared to 60 Gy in three fractions (utilized in RTOG 0236).
Lung Metastases
Since Halsted’s initial description of the contiguous spread of cancer, data illustrating long term, disease-free survival in patients with metastases led to Hellman’s proposed theory of oligometastases (76,77). His theory described a disease state with a limited number of metastases that had not evolved to widespread disease (78). If this is the case, one could implement aggressive local therapy as curative-intent treatment for oligometastases. Surgical resection data for pulmonary metastases have illustrated improvements in survival, with 5- and 10-year overall survival (OS) of 36% and 25%, respectively (79). Based on this improvement in survival with aggressive local control, radiotherapy has also been explored for select patients. In addition, given the success with SBRT in the treatment of primary lung tumors, this technique is now being applied in the oligometastatic setting (32,69,70,71,72,73,74,75,80).
Phase II data from Rochester illustrated that 49 patients with 121 metastatic lung lesions had a 3-year actuarial local control rate of 91%. Mean overall survival was 23.4 months and treatment was tolerated well, with 2% grade 3 toxicity (81). Data from Germany showed that 61 patients with lung metastases treated for 71 lesions with SBRT had a 2-year actuarial local control rate and overall survival rate of 74% and 65%, respectively (82). Hoyer et al. published phase II data assessing SBRT in colorectal metastasis with 12 patients with pulmonary metastases treated to 45 Gy in three fractions. Two year actuarial local control was 86% (83).
Recently, a multi-institutional, prospective phase I/II trial evaluating SBRT in patients with 1–3 lung metastases treated to 60 Gy illustrated an actuarial 2-year local control rate of 96%, median survival of 19 months, and grade 3 toxicity rate of 8% (84). In the oligometastatic setting, these studies suggest that SBRT is both well tolerated and effective, with results comparable to metastasectomy, and could be a valuable noninvasive treatment option for patients.
Lung SBRT Side Effects
Side effects from stereotactic radiation treatment can include radiation pneumonitis, bronchial injury, pulmonary function test changes, rib pain, and dermatologic changes. Radiation pneumonitis is an inflammation of the bronchioles and alveoli that can occur weeks to months after treatment. The clinical presentation can be similar to an acute bacterial pneumonia and includes a nonproductive cough, chest pain, shortness of breath, and fever. Chest X-ray typically illustrates an area of consolidation that can correlate with regions of high dose. Typical treatment includes nonsteroidal anti-inflammatory agents, steroid inhalers, systemic steroids, bronchodilators, and pulmonary toilet. In immunocompromised patients, bactrim can be used to avoid opportunistic infections. It is important to consider GI prophylaxis and monitoring of blood sugar levels in patients receiving steroids.
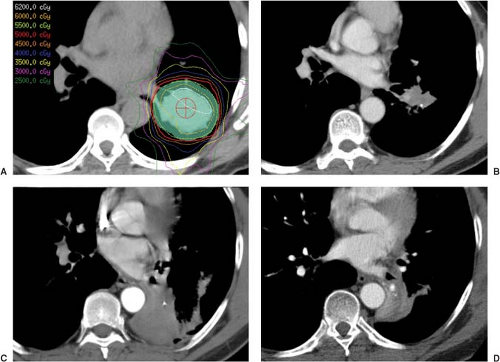 Figure 16.3. Stereotactic body radiation therapy (SBRT) of a central lung tumor treated on protocol. Panel A shows the isodose plan. While follow-up scans are shown at 3 months, 6 months, and 1 year in panel B, C, and D respectively. There was an excellent initial response with eventual collapse of the downstream lung.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|