Highlights
- •
Observational studies suggest favorable associations between statin use and prostate cancer (CaP) outcomes, data from randomized-controlled trials remain inconclusive.
- •
We explored the relationship between statin use and survival outcomes in the context of the phase III ARAMIS study, a trial of darolutamide in the treatment of nonmetastatic castration-resistant prostate cancer.
- •
In our secondary analysis of the ARAMIS trial, statin users had similar metastasis-free survival and secondary outcomes compared to nonusers.
- •
Pursuing further statin synergies with amide-based androgen receptor axis target agents may not be fruitful.
Abstract
Introduction
While observational studies suggest favorable associations between statin use and prostate cancer (CaP) outcomes, data from randomized-controlled trials remain inconclusive. Our study explores the relationship between statin use and survival outcomes in the context of the phase III ARAMIS study, a trial of darolutamide in the treatment of nonmetastatic castration-resistant prostate cancer.
Methods
We reviewed all 1,509 patients in the ARAMIS trial. Statin use was identified at baseline. Statin users were matched 1:2 with nonusers using a propensity score matching model. The primary endpoint was metastasis-free survival (MFS). Kaplan-Meier curves were plotted for MFS comparing statin users and nonusers across ARAMIS trial arms. A multivariate Cox proportional hazards model was fitted using the propensity-matched cohort and incorporating statin use and all covariates.
Results
Of the 1,509 patients in ARAMIS, 334 (22.1%) were statin users. We matched 297 statin users to 550 nonusers. Characteristics appeared well balanced. Among nonusers, 331 (60.3%) and 219 (39.7%) were in the ARAMIS darolutamide and placebo arms, respectively. Among statin users, 179 (60.3%) and 118 (39.7%) were in the ARAMIS darolutamide and placebo arms, respectively. Overall, we found no significant difference in MFS between statin users and nonusers (HR 1.05, 95% CI 0.80–1.38 P = .72). However, we found significant interaction between statin use and ARAMIS trial arm. Specifically, statin use had a stronger association with MFS in the placebo arm ( P = 0.024). However, this is likely coincidental and due to the statin-placebo patients having higher nodal positivity than the nonusers-placebo patients (14.3% vs. 5.5%). Statin use was similarly not associated with the secondary outcomes of PSA progression-free survival ( P = 0.42), time-to-pain progression ( P = 0.85), or overall survival ( P = 0.15).
Conclusions
In our secondary analysis of the ARAMIS trial, statin users had similar MFS and secondary outcomes compared to nonusers. These results suggest pursuing further statin synergies with amide-based androgen receptor axis target agents may not be fruitful.
1
Introduction
There is increasing interest in statin medications in prostate cancer (PCa). Studies have shown favourable associations between statin use and CaP outcomes. Statins may selectively reduce the risk of advanced and lethal prostate cancer [ ], prolong time to disease progression [ ], and improve survival [ , , ]. Unfortunately, randomized-controlled trials supporting a statin anticancer effect are lacking or conflicting [ , ]. In very advanced settings, such as metastatic castrate resistant prostate cancer (CRPC), the data are contradictory. For example, in a large retrospective study of mCRPC patients receiving abiraterone or enzalutamide after treatment with docetaxel, a positive association between statin use and overall- and cancer specific- survival was observed [ ]. However, in 2 other trials the authors did not observe an OS benefit associated with statin use among mCRPC patients treated with Abiraterone [ , ]. It is possible that in earlier disease settings, where survival times are longer, more of a benefit attributable to statin use would be observed. For example, in our secondary analysis of the PR7 randomized trial of intermittent vs. continuous ADT in men with biochemical recurrence, statin use was associated with a reduced risk of overall (HR: 0.64; 95% C.I. 0.53–0.78, P < 0.001) and CaP-specific mortality (HR: 0.63, 95% C.I. 0.47–0.85, P = 0.002) [ ].
The ARAMIS was a phase 3 trial involving 1,509 men with nonmetastatic castration-resistant prostate cancer (nmCRPC). Darolutamide, an androgen-receptor pathway inhibitor (ARPI) demonstrated significant efficacy in delaying metastasis and death with a median metastasis-free survival of 40.4 months, compared to 18.4 months with placebo. Darolutamide also improved overall survival, with a 31% reduction in the risk of death (HR 0.69; 95% CI, 0.53–0.88; P = 0.003) [ ], time to pain progression, time to cytotoxic chemotherapy, and time to symptomatic skeletal events. Strengths of darolutamide include the reduced adverse effects as theoretically its active metabolite has low penetration of the blood brain barrier (BBB) [ ], and a limited potential for clinically relevant drug-drug interactions (DDIs) [ ].
To date, synergies with statin use have not been studied in a randomized trial such as ARAMIS where darolutamide the newest and least toxic ARPI was used to delay metastasis in patients with nmCRPC. The aim of this study is to assess, the association between statin use at baseline and prostate cancer outcomes.
2
Material and methods
2.1
Trial design
The phase III ARAMIS trial (NCT02200614) protocol and design have been described in detail elsewhere [ ]. Briefly, this was a randomized, double-blind, placebo-controlled, phase III clinical trial conducted globally in 409 centers in 36 countries to evaluate the efficacy and safety of darolutamide in patients with nmCRPC. Men were randomized 2:1 to receive darolutamide 600 mg twice daily (given as two 300 mg tablets) or placebo while continuing ADT.
2.2
Participants and variables
After REB approval (CAPCR # 23-5937) and approval from Bayer Clinical Study Data Request consortium (CSDR) we reviewed all 1,509 patients in the intention-to-treat population (ITT) from the ARAMIS trial [ , ]. We identified statin use at baseline and we used a propensity score matching model using the following variable of interests to match 2 nonusers to 1 statin user. The variables of interest used for propensity score matching were: Age (categorized as <65, 65–74, 75–84, ≥85), race, ECOG (0 vs. 1), PSA at baseline, PSA doubling time, presence of lymph nodes (N0 vs. N1), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), hemoglobin, testosterone (baseline), neutrophil to lymphocyte ratio (NLR), time with castration resistant prostate cancer (CRPC) in months, opioid use, use of bone sparing agents, previous androgen deprivation therapy (ADT), and region (North America, Asia-pacific, rest of the world). Testosterone, NLR, ALP and LDH were log-transformed to improve model fit.
2.3
End points and assessments
The primary endpoint was to assess the association between statin use and metastases-free survival (MFS) as defined in the ARAMIS trial [ , ]. Secondary endpoints were: Overall survival (OS), time to pain progression (TTP), Progression-free survival (PFS), and time to PSA progression, all of them as defined in the ARAMIS trial [ , ].
2.4
Statistical analysis
Descriptive statistics were used to summarize patient characteristics. Quantitative variables have been summarized through measures of central tendency (mean and median), and dispersion (standard deviation and interquartile range). Qualitative variables are outlined with measurements of absolute and relative frequencies. Using all confounders listed previously, we calculated a propensity score for statin use using a logistic regression model. For each statin user, we match 2 nonusers using the nearest neighbor matching algorithm with a caliper width of 0.2 times the standard deviation of the propensity scores. To ensure the cohort was well-matched, we plotted the propensity score distribution between the statin and nonusers and review the standardized differences of each characteristic between statin and nonusers. We forced an exact match on treatment (darolutamide vs. placebo). To assess the impact of statin use for the primary and secondary outcomes, we plotted 2 Kaplan-Meier curves for each outcome, the first stratifying results by statin use, and the second stratifying results by statin use and treatment arm in the ARAMIS trial (darolutamide vs. placebo). We used the log-rank test to compare the survival curves. Additionally, a multivariate Cox proportional hazards model was fitted for each outcome using the propensity-matched cohort and incorporating statin use and all covariates incorporated in the propensity score matching (PSM) model. A sensitivity analysis using treatment arm as a matching variable was performed and this new matched cohort was analyzed for each outcome (MFS, PFS, time to PSA progression, time to pain progression, OS). Additionally, statin type and dose effect on all outcomes was tested in this cohort. All analyses were 2-sided, with P < 0.05 considered statistically significant. Statistical analyses were conducted using R ( www.R-project.org ) and SAS® version 9.4.
3
Results
A total of 1,509 patients were randomized in the ARAMIS trial. Of these, 334 patients were statin users at baseline, 997 were nonusers, and 178 had missing information or started to receive statins after enrollment. During matching, 11 patients were excluded because of missing clinical data. The total number of patients eligible for the matched cohort was n = 1,320 (n = 332 statin users, n = 988 nonusers). A total of 297 statin users were matched to 550 nonusers and will be the subject of the remaining analyses ( Fig. 1 ).
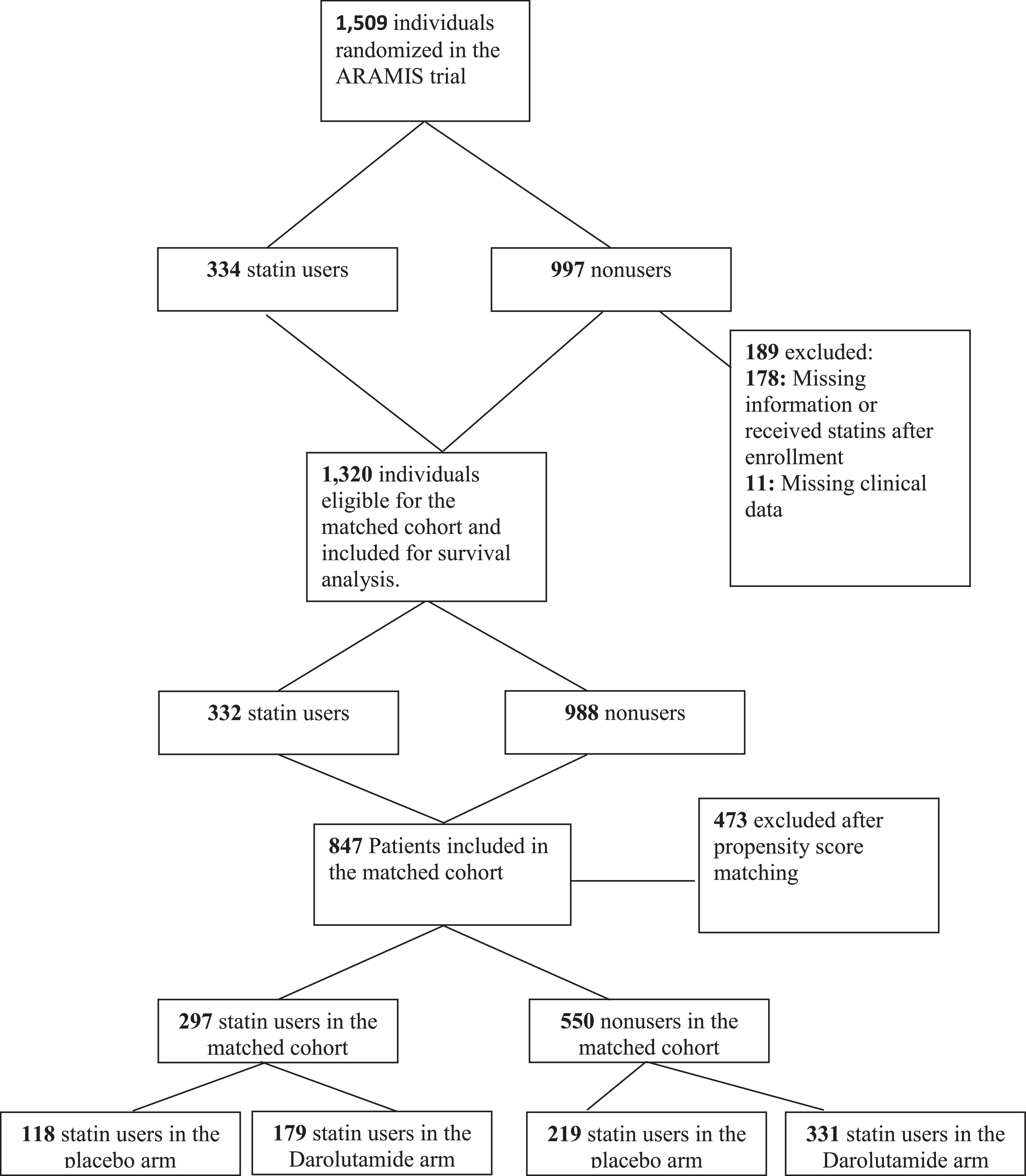
The characteristics of the cohort appeared well-balanced ( Table 1 ) with the exception of distribution of statin users across ARAMIS trial arms. Table 2 displays baseline characteristics stratified by both statin use and ARAMIS arm. The 4 groups appeared well-balanced with the exception of nodal status, where more statin users in the placebo group had nearly 3 times the presence of nodal disease (12.5% vs. 5.6%, P = 0.099).
| Characteristic | Overall N = 847 | Statin Nonusers N = 550 | Statin Users N = 297 | Standardized Mean Difference |
|---|---|---|---|---|
| Treatment | 0.00 | |||
| Placebo | 337 (39.7%) | 219 (39.7%) | 118 (39.7%) | |
| Darolutamide | 510 (60.3%) | 331 (60.3%) | 179 (60.3%) | |
| Statin type | 0.00 | |||
| Hydrophilic | 225 (26.5%) | – | 225 (75.8% | |
| Lipophilic | 72 (8.5%) | – | 72 (24.2%) | |
| Age | 0.03 | |||
| <65 | 83 (9.8%) | 53 (9.6%) | 30 (10.1%) | |
| 65-74 | 325 (38.4%) | 211 (38.4%) | 114 (38.4%) | |
| 75-84 | 356 (42.0%) | 231 (41.9%) | 125 (42.1%) | |
| ≥85 | 84 (9.9%) | 56 (10.1%) | 28 (9.4%) | |
| Race | 0.03 | |||
| Asian | 95 (11.2%) | 62 (11.3%) | 33 (11.1%) | |
| Black or African American | 36 (4.3%) | 23 (4.2%) | 13 (4.4%) | |
| Other | 26 (3.0%) | 16 (2.9%) | 10 (3.4%) | |
| White | 690 (81.5%) | 449 (81.6%) | 241 (81.1%) | |
| ECOG | 0.03 | |||
| 0 | 572 (67.5%) | 374 (68.0%) | 198 (66.7%) | |
| 1 | 275 (32.5%) | 176 (32.0%) | 99 (33.3%) | |
| Region | 0.00 | |||
| Asia Pacific | 91 (10.8%) | 59 (10.8%) | 32 (10.8%) | |
| North America | 160 (18.9%) | 104 (18.9%) | 56 (18.9%) | |
| Rest of the World | 596 (70.4%) | 387 (70.4%) | 209 (70.4%) | |
| Baseline PSA | 0.05 | |||
| ≤10 | 451 (53.2%) | 288 (52.4%) | 163 (54.9%) | |
| >10 to ≤20 | 191 (22.5%) | 127 (23.1%) | 64 (21.5%) | |
| >20 | 205 (24.2%) | 135 (24.6%) | 70 (23.6%) | |
| PSA Doubling Time | 0.03 | |||
| ≤6 mo | 570 (67.3%) | 373 (67.8%) | 197 (66.3%) | |
| >6 mo | 277 (32.7%) | 177 (32.2%) | 100 (33.7%) | |
| Presence of Lymph Nodes | 74 (8.7%) | 49 (8.9%) | 25 (8.4%) | 0.02 |
| ALP | 0.00 | |||
| Mean (SD) | 78.79 (23.58) | 78.75 (22.90) | 78.85 (24.83) | |
| Median (IQR) | 75.00 (62.00, 90.00) | 75.00 (63.00, 90.00) | 73.50 (61.00, 90.00) | |
| Range | 23.00, 223.00 | 26.00, 223.00 | 23.00, 179.00 | |
| LDH | 0.03 | |||
| Mean (SD) | 186.30 (42.68) | 186.71 (46.48) | 185.54 (34.59) | |
| Median (IQR) | 181.00 (161.00, 206.00) | 180.00 (162.00, 205.00) | 182.00 (161.00, 209.00) | |
| Range | 89.00, 908.00 | 89.00, 908.00 | 93.00, 326.00 | |
| HGB | −0.02 | |||
| Mean (SD) | 13.00 (1.25) | 12.99 (1.29) | 13.02 (1.17) | |
| Median (IQR) | 13.10 (12.20, 13.80) | 13.10 (12.20, 13.80) | 13.05 (12.40, 13.70) | |
| Range | 7.10, 16.80 | 7.10, 16.70 | 9.90, 16.80 | |
| Testosterone | −0.02 | |||
| Mean (SD) | 0.60 (0.34) | 0.59 (0.24) | 0.60 (0.46) | |
| Median (IQR) | 0.53 (0.46, 0.69) | 0.54 (0.46, 0.70) | 0.52 (0.46, 0.67) | |
| Range | 0.16, 7.25 | 0.16, 1.96 | 0.16, 7.25 | |
| Neutrophil to Lymphocyte Ratio | −0.09 | |||
| Mean (SD) | 2.84 (1.43) | 2.79 (1.36) | 2.92 (1.54) | |
| Median (IQR) | 2.55 (1.93, 3.37) | 2.52 (1.95, 3.27) | 2.56 (1.92, 3.59) | |
| Range | 0.40, 14.74 | 0.58, 14.74 | 0.40, 13.24 | |
| # Mo castrate-sensitive disease | 0.00 | |||
| <6 mo | 405 (47.8%) | 263 (47.8%) | 142 (47.8%) | |
| 6-12 mo | 229 (27.0%) | 149 (27.1%) | 80 (26.9%) | |
| >12 mo | 213 (25.1%) | 138 (25.1%) | 75 (25.3%) | |
| Opioid | 28 (3.3%) | 17 (3.0%) | 11 (3.7%) | 0.04 |
| Bone Sparing Agent | 39 (4.6%) | 24 (4.4%) | 15 (5.1%) | 0.03 |
| Previous Hormone Therapy | 819 (96.7%) | 532 (96.8%) | 287 (96.6%) | 0.01 |
| Characteristic | Statin NonUsers, Darolutamide N = 331 | Statin NonUsers, Placebo N = 219 | Statin Users, Darolutamide N = 179 | Statin Users, Placebo N = 118 | P -value |
|---|---|---|---|---|---|
| Age | 0.43 | ||||
| <65 | 25 (7.5%) | 28 (12.7%) | 13 (7.3%) | 17 (14.4%) | |
| 65-74 | 125 (37.7%) | 86 (39.4%) | 72 (40.2%) | 42 (35.6%) | |
| 75-84 | 146 (44.1%) | 84 (38.6%) | 76 (42.5%) | 49 (41.5%) | |
| ≥85 | 35 (10.6%) | 20 (9.3%) | 18 (10.1%) | 10 (8.5%) | |
| Race | 0.51 | ||||
| Asian | 34 (10.3%) | 28 (12.7%) | 18 (10.1%) | 15 (12.7%) | |
| Black or African American | 12 (3.6%) | 11 (5.1%) | 7 (3.9%) | 6 (5.1%) | |
| Other | 12 (3.6%) | 4 (1.7%) | 3 (1.7%) | 7 (5.9%) | |
| White | 273 (82.4%) | 176 (80.5%) | 151 (84.4%) | 90 (76.3%) | |
| ECOG | 0.48 | ||||
| 0 | 219 (65.9%) | 156 (71.2%) | 116 (64.8%) | 82 (69.5%) | |
| 1 | 113 (34.1%) | 63 (28.8%) | 63 (35.2%) | 36 (30.5%) | |
| Region | >0.99 | ||||
| Asia Pacific | 33 (10.1%) | 26 (11.9%) | 18 (10.1%) | 14 (11.9%) | |
| North America | 63 (19.0%) | 41 (18.6%) | 34 (19.0%) | 22 (18.6%) | |
| Rest of the World | 235 (70.9%) | 152 (69.5%) | 127 (70.9%) | 82 (69.5%) | |
| Baseline PSA | 0.68 | ||||
| ≤10 | 175 (52.8%) | 113 (51.7%) | 106 (59.2%) | 57 (48.3%) | |
| >10 to ≤20 | 76 (22.9%) | 51 (23.3%) | 36 (20.1%) | 28 (23.7%) | |
| >20 | 81 (24.3%) | 55 (25.0%) | 37 (20.7%) | 33 (28.0%) | |
| PSA Doubling Time | 0.97 | ||||
| ≤6 mo | 226 (68.2%) | 147 (67.4%) | 119 (66.5%) | 78 (66.1%) | |
| >6 mo | 106 (31.8%) | 71 (32.6%) | 60 (33.5%) | 40 (33.9%) | |
| Presence of Lymph Nodes | 25 (7.5%) | 24 (11.0%) | 10 (5.6%) | 15 (12.7%) | 0.099 |
| ALP | 0.13 | ||||
| Mean (SD) | 76.44 (20.51) | 82.26 (25.76) | 78.69 (24.21) | 79.09 (25.84) | |
| Median (IQR) | 73.00 (62.00, 88.00) | 77.00 (65.00, 91.00) | 73.00 (60.00, 93.25) | 76.00 (62.50, 87.00) | |
| Range | 35.00, 176.00 | 26.00, 223.00 | 23.00, 144.00 | 37.00, 179.00 | |
| LDH | 0.50 | ||||
| Mean (SD) | 186.95 (51.99) | 186.33 (36.70) | 187.97 (35.56) | 181.86 (32.87) | |
| Median (IQR) | 180.00 (160.00, 205.00) | 182.00 (164.00, 202.00) | 184.00 (161.00, 213.00) | 178.00 (157.00, 204.00) | |
| Range | 90.00, 908.00 | 89.00, 359.00 | 93.00, 326.00 | 110.00, 283.00 | |
| HGB | 0.88 | ||||
| Mean (SD) | 13.02 (1.27) | 12.95 (1.31) | 12.97 (1.13) | 13.09 (1.23) | |
| Median (IQR) | 13.10 (12.25, 13.80) | 13.10 (12.20, 13.80) | 13.00 (12.20, 13.70) | 13.10 (12.40, 13.85) | |
| Range | 7.10, 16.70 | 7.10, 16.70 | 10.00, 16.10 | 9.90, 16.80 | |
| Testosterone | 0.17 | ||||
| Mean (SD) | 0.58 (0.24) | 0.61 (0.24) | 0.57 (0.21) | 0.65 (0.68) | |
| Median (IQR) | 0.53 (0.45, 0.68) | 0.55 (0.47, 0.74) | 0.52 (0.46, 0.66) | 0.53 (0.45, 0.69) | |
| Range | 0.17, 1.82 | 0.16, 1.96 | 0.16, 1.42 | 0.17, 7.25 | |
| Neutrophil to Lymphocyte Ratio | 0.19 | ||||
| Mean (SD) | 2.75 (1.22) | 2.86 (1.55) | 2.78 (1.38) | 3.14 (1.75) | |
| Median (IQR) | 2.52 (1.93, 3.28) | 2.52 (1.97, 3.25) | 2.41 (1.89, 3.38) | 2.82 (1.98, 3.81) | |
| Range | 0.58, 9.93 | 0.68, 14.74 | 0.40, 10.35 | 0.51, 13.24 | |
| # Mo castrate-sensitive disease | 0.74 | ||||
| <6 mo | 153 (46.1%) | 110 (50.4%) | 85 (47.5%) | 57 (48.3%) | |
| 6-12 mo | 87 (26.3%) | 62 (28.4%) | 51 (28.5%) | 29 (24.6%) | |
| >12 mo | 92 (27.7%) | 46 (21.2%) | 43 (24.0%) | 32 (27.1%) | |
| ) | 0.12 | ||||
| Bone Sparing Agent | 14 (4.2%) | 10 (4.7%) | 7 (3.9%) | 8 (6.8%) | 0.71 |
| Previous Hormone Therapy | 325 (98.0%) | 207 (94.9%) | 173 (96.6%) | 114 (96.6%) | 0.24 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree