Abstract
This review assesses the current understanding of the relationship between the human microbiome and BCa. Recognizing how the microbiome affects the tumor microenvironment provides valuable insights into cancer biology, potentially uncovering interactions that could be leveraged to develop innovative therapeutic approaches. By clarifying these intricate microbial-tumor dynamics, novel targets for microbiome-based interventions can be identified, ultimately improving treatment effectiveness and patient outcomes. Current literature lacks comprehensive insights into the effects of BCa treatment on the microbiome and the prevalence of immunotherapy-related toxicities. Further research into the microbiome’s role in BCa development could bridge the gap between fundamental research and therapeutic applications. Implementing microbiome surveillance, metagenomic sequencing, and metabolomics in clinical trials could deepen our understanding of BCa and its treatment. This review explores the existing understanding of the urine, tissue, and gut microbiomes and their connections to BCa. Enhanced knowledge of these relationships can pave the way for future research to identify reliable disease predictors, prognostic markers, and novel therapeutic targets.
1
Introduction
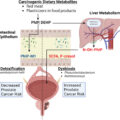
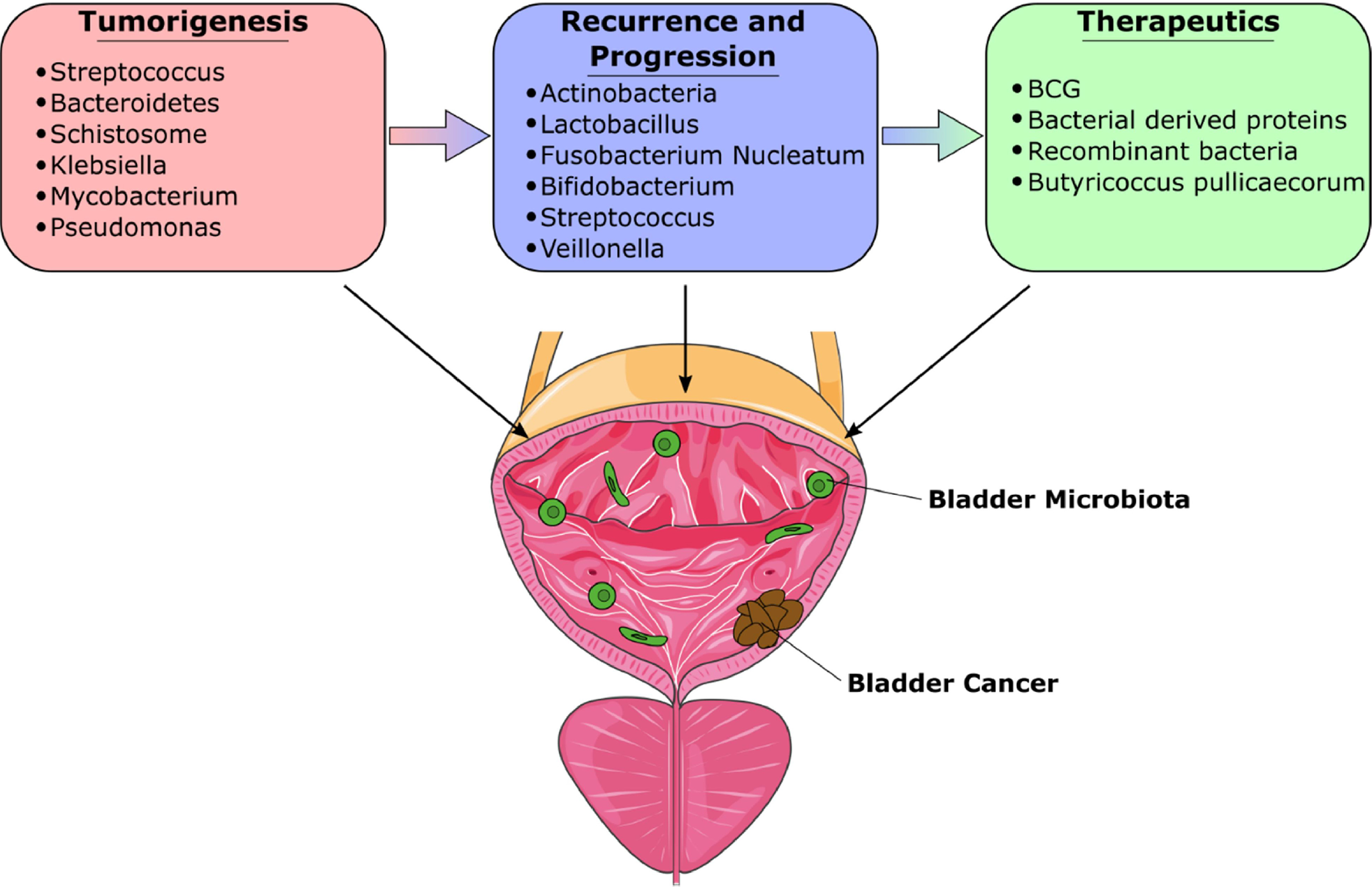
Over the past decade, there has been increasing recognition of the microbiome’s crucial role in bladder cancer (BCa), particularly in its development and treatment [ , ]. This interest originates from studies that show that various environmental and genetic factors, including geographic location, food habits, sexual activity, and anatomical variances, can lead to microbiome fluctuation, immune maturation, and even development of disease [ ]. Research has highlighted mechanisms by which specific bacterial species contribute to BCa development. These bacteria produce proteases that can damage host tissues, compromise immune defenses, and disrupt physical barriers. [ ]. Such activities may foster BCa growth by triggering inflammation, changes in the extracellular matrix (ECM), and generation of oxygen radicals [ ], creating conditions conducive to mutagenesis. With an emphasis on the possibility of utilizing the microbiome for therapeutic interventions, this review aims to explore the growing evidence of knowledge about the role of the urine, tissue, and gut microbiome in the development of BCa ( Fig. 1 ). Fig 2 .
2
Literature review
We conducted a narrative review of the existing research on the relationship between the microbiome and BCa up to March 2024. Our review focused on peer-reviewed research studies and review articles, utilizing databases such as PubMed and Google Scholar for source material. We employed search terms including “microbiome,” “microbiota,” “urinary microbiome,” “gut microbiome,” “urothelial carcinoma,” “intratumoral microbiota,” and “bladder cancer” to ensure a thorough exploration of relevant literature. In total, 360 articles were initially identified through our search. We applied inclusion criteria such as relevance to microbiome-BCa relationships, focusing on studies that provided empirical data or hypotheses on microbial diversity or specific populations linked to tumorigenesis, progression, or therapeutic outcomes. Studies were excluded if they focused on non-urothelial cancers, lacked supporting data, or did not directly address microbiome-bladder cancer interactions. To minimize selection bias, given the tendency for studies with positive findings or novel associations to be overrepresented, we sought a balanced perspective by including research addressing both significant findings and negative results, especially considering the limited research on less-studied sites such as the bladder microbiome. As this is a narrative review, our synthesis of the included studies is thematic, emphasizing understanding and interpreting the complex interactions between different microbiome types and BCa development rather than aggregating quantitative data from multiple studies as done in a systematic review.
3
Recent trends in microbiome research in bladder cancer
3.1
Urinary microbiome
3.1.1
Impact of the urinary microbiome on BCa development
It is unclear whether the urinary tract microbiome affects the development or progression of BCa or if cancer impacts the bladder microbiome. One theory is that the bladder microbiome alters ECM, which may inhibit or promote inflammation and urothelial cell carcinogenesis [ ].
A study by Popović et al . [ ] investigated urinary microbiome in BCa patients ( n = 12) compared to healthy controls ( n = 11). They found no significant differences in microbial diversity or overall composition between the groups. The Firmicutes phylum was the most common in both groups. Despite the structural similarities, certain bacteria, such as Fusobacterium were more abundant in BCa patients, indicating a possible association with carcinogenesis. Additionally, Campylobacter hominins were notably prevalent in BCa patients, suggesting a potential link to BCa [ ].
Bi et al . [ ] conducted a study involving urine samples from 29 BCa patients and 26 controls, identifying a core microbiome composed of 4 phyla: Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes. Across all samples, 5 bacterial genera- Streptococcus, Bifidobacterium, Lactobacillus, Veillonella, and Actinomyces were consistently present in all samples. However, significant differences were observed between the BCa and control groups, with Actinomyces displaying a higher abundance in BCa patients than healthy controls ( P < 0.05).
3.1.2
Smoking and urinary microbiome
Ma et al. [ ] conducted a study to assess the impact of smoking on the urinary microbiome in male patients with BCa. They examined 26 urine samples, including 15 from men with BCa and 11 from healthy men. Their analysis revealed that certain bacterial families, such as Bacteroidaceae, Erysipelotrichales, and Lachnospiraceae, were more abundant in smokers. Principal component analysis (PCA), a dimensionality reduction technique, revealed a significant distinction between smokers and nonsmokers with BCa ( P < 0.001), while alpha diversity, a measure of microbial richness and diversity within a sample, was also higher among smokers ( P < 0.05). Additionally, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis indicated that smokers demonstrated increased metabolic activity compared to nonsmokers, including pathways linked to metabolic diseases, glycan biosynthesis and metabolism, and nucleotide metabolism. Smokers also had heightened activity in pathways related to the immune system and inflammation, which could contribute to chronic inflammation or immune suppression. This altered metabolic activity may exacerbate conditions conducive to cancer progression by creating a microenvironment that promotes tumor growth.
A recent study by Bukavina et al. [ ] assessed the urinary microbiome in BCa patients globally, comparing it with noncancer controls while evaluating the confounding influences of environmental factors versus associations with bladder cancer. The research encompassed 129 BCa patients and 60 healthy controls across 4 countries and 3 continents. Out of 548 bacterial genera analyzed, 97 were found to be differentially abundant in the urine of BCa patients compared to healthy individuals. A significant finding of the study was that many of the observed taxonomic differences could be attributed to the urine collection methods (voided vs. catheterized). Furthermore, the study identified a notable presence of polycyclic aromatic hydrocarbon (PAH)-degrading bacteria, such as Sphingomonas, Acinetobacter, Micrococcus, Pseudomonas, and Ralstonia , which were consistently more abundant in BCa patients after accounting for contaminants associated with voided urine. This suggests a potential biomarker or a microbial influence on the disease pathway.
3.1.3
Microbiome diversity and BCa risk stratification
Wu et al. [ ] analyzed mid-stream urine samples from 31 male BCa patients and 18 controls. They revealed a statistically significant difference in beta diversity between the groups BCa ( P < 0.05), while no significant variation was observed among different tumor grades. Specifically, authors found genera Acinetobacter and Anaerococcus to be significantly more abundant in male Chinese BCa patients compared to healthy controls ( Acinetobacter : BCa vs. controls, 3.25% vs. 1.09%, P < 0.05; Anaerococcus : BCa vs. controls, 9.28% vs. 4.59%, P < 0.05). Moreover, an enrichment of Herbaspirillum, Porphyrobacter, and Bacteroides was observed in BCa patients with a high risk of recurrence and progression, which suggests that these genera may be potential biomarkers for risk stratification and predict cancer prognosis. These findings underscore the potential of these microbial markers in risk stratification among BCa patients. The study’s scope was constrained by several limitations, including its small sample size, utilization of midstream collection, absence of decontamination protocols, and insufficient testing for confounding variables. Likewise, Zeng et al. [ ] also examined urinary microbiome as a potential predictor of recurrence. The study identified nine specific genera that were disproportionately prevalent in patients experiencing recurrence, including Anoxybacillus, Massilia, Thermomonas, Brachybacterium, Micrococcus, Nocardioides, Larkinella, Jeotgalibacillus, and Geomicrobium (with an LDA) threshold >2.5). Nonetheless, similar to the study conducted by Wu et al. [ ] , this analysis was constrained by a limited sample size, and it is suggested that many of the aforementioned bacteria might be sample contaminants rather than genuinely representative genera.
3.1.4
Gender differences in BCa urinary microbiome
Gender differences in the urinary microbiome of BCa patients are a crucial area of interest due to their potential impact on disease progression and therapeutic approaches. In this context, Hussein et al. [ ] found that male BCa patients had a higher abundance of Pelomonas, Finegoldia, and Corynebacterium in their urobiome, while female patients had a greater prevalence of Enterococcus, Campylobacter, and Ruminococcocae . Similarly, Pederzoli et al. [ ] analyzed 16S rDNA sequences in urine and tissue samples, confirming the presence of a gender-specific BCa microbiome. They also noted an enrichment of Klebsiella species in the urine of female patients, linking it with the release of colibactin toxin, which can induce DNA damage and genomic instability [ ]. This study’s tissue microbiome analysis revealed a Burkholderia predominance among BCa patients, although no significant gender-based differences were observed. Hourigan et al . [ ] further explored gender disparities by analyzing urine samples collected through voided and cystoscopic methods. They observed differences in beta diversity between these collection methods in male samples, but not in female samples, revealing a potential methodological factor that could influence future research on BCa urobiome composition. Moreover, all three studies emphasized Burkholderia’s predominance in neoplastic bladder tissues, suggesting a potential role in BCa pathogenesis regardless of gender. These results should be interpreted cautiously because Burkholderia is often a contaminant in water, soil, and common reagents. It is frequently identified as a contaminant across laboratories due to varying kit batches and extraction kits [ ].
Another study by Fouts et al . [ ] used 16S rRNA sequencing to identify a predominance of Lactobacillales species in females and Corynebacterium in males. They found that Lactobacillus species, which produce lactic acid, play a protective role against urinary tract infections, highlighting another layer of complexity in gender-based differences. Although data remains limited, these results emphasize the intricate interplay between gender and the urobiome in BCa patients, indicating that the urobiome’s composition is not only affected by the disease but also shows distinct patterns based on the patient’s gender.
3.1.5
Urobiome variations and their correlation with BCa progression and treatment responses
A study by Chipollini et al. [ ] analyzed three groups: a control group ( n = 10), a group with muscle-invasive bladder cancer (MIBC, n = 15), and a group with superficial BCa ( n = 12). Their results showed that Bacteroides and Faecalibacterium were particularly enriched in invasive BCa samples, aligning with findings from Popović et al. [ ].
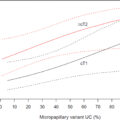
Wu et al. [ ] offered additional findings, noting that 6 genera were overrepresented in patients at high risk of recurrence (HER) and four genera in those at high risk of progression (HEP). The HER group had higher levels of Herbaspirillum, Gemella, Bacteroides, Porphyrobacter, Faecalibacterium, and Aeromonas . Meanwhile, the HEP group showed increased abundances of Herbaspirillum, Porphyrobacter, Bacteroides, and Marmoricola . Overall, their study also found that Haemophilus, Veillonella, and Fusobacterium were more prevalent in BCa patients, particularly those with muscle-invasive disease Fig 2 .
Similarities between Wu et al. [ ] and Hussein et al. [ ] found Haemophilus and Veillonella to be more common in patients with MIBC indicating consistent stage-specific patterns. Furthermore, Hussein et al . [ ] discovered that Serratia and Brochothrix were more prevalent in NMIBC patients who responded well to Bacillus Calmette-Guérin (BCG) therapy, suggesting a potential connection between urobiome composition and treatment outcomes. Together, these studies highlight commonalities in the BCa microbiome while identifying unique bacterial profiles associated with different cancer stages and treatment responses. It remains uncertain whether the microenvironment within high-stage tumors promotes the specialization of bacteria or if the presence of certain bacteria contributes to the upstaging of disease by causing local tissue destruction and immunomodulation. Since many studies focused solely on characterization without further culturomics or follow-up experiments focusing on mechanism of possible tissue destruction or immune modulation, these associations remain speculative and have not yet demonstrated true causality.
On the contrary, Qiu et al. [ ] evaluated the potential influence of urinary bacterial composition on immune responses and cancer recurrence in patients diagnosed with nonmuscle invasive bladder cancer (NMIBC). Their study involved detailed assessments of the presence of FoxP3+ regulatory T cells within tumors, Ki-67 protein expression levels, and the likelihood of cancer recurrence in relation to the types of bacteria found in urine. The analysis revealed significant associations between urinary bacterial profiles and clinical outcomes in NMIBC patients. Specifically, individuals who experienced cancer recurrence exhibited notably higher levels of certain bacterial species in their urine samples, including P seudomonas, Staphylococcus, Corynebacterium, and Acinetobacter . Furthermore, the authors found that patients with a greater variety of bacteria in their urine had elevated levels of Ki-67 expression, indicating increased cellular proliferation within tumor tissues ( P = 0.0194). However, no significant difference was observed in the presence of regulatory T cells between individuals with varying degrees of urinary bacterial diversity ( P = 0.1653). Of clinical relevance, the study highlighted that patients with less diverse urinary bacterial populations experienced longer intervals without cancer recurrence compared to those with a more diverse bacterial composition.
3.2
Intratumoral microbiota associated with bladder cancer
There exists a significant gap in the research pertaining to the intratumoral microbiota associated with BCa. The majority of existing studies have mainly focused on analyzing urinary samples from BCa patients to understand the microbiome’s role and characteristics. This approach, while valuable, leaves an unexplored area in the direct examination of the bladder tissue microbiome, which could potentially offer deeper insights into the disease’s pathology and tumor microenvironment interactions at the site of the cancer itself.
3.2.1
Tissue-specific microbiome expression
In a 2019 study by Liu et al. [ ] researched aimed to compare tissue specific differences in microbiome, evaluating differences between cancerous and noncancerous states. By examining 12 samples of BCa tumor tissue alongside corresponding normal tissue specimens, they uncovered a significant discrepancy in the Shannon diversity index, a metric indicating species diversity, with tumor tissue exhibiting notably lower diversity compared to its noncancerous counterpart. Intriguingly, the analysis revealed decreased relative abundances of microbial genera such as Lactobacillus, Prevotella_9, and Ruminococcaceae within the cancerous tissues, and an increased abundance of Cupriavidus spp ., as well as Acinetobacter, Anoxybacillus, Escherichia-Shigella, and Sphingomonas. These findings echo previous observations from urine studies, suggesting a correlation between the microbial composition of bladder tissue and urine. Specifically, the increased presence of Acinetobacter in cancerous tissue and the potential protective role of Lactobacillus against bladder cancer tumorigenesis have been consistent findings across both tissue and urine analyses.
Using TCGA data, Rodrigues et al. [ ] conducted a comparison of 56 paired tumor and adjacent normal samples from 28 MIBC cases. Upon analyzing all available tumor and adjacent normal files, Mathylobacterium radiotolerans, Pseudomonas aeruginosa, and Pseudomonas putida were identified as differentially abundant in tumor compared to normal tissue. However, subsequent analysis using a fully adjusted model did not reveal any statistically significant differences in abundance between the 2 groups.
While Rodrigues et al. [ ] did not identify a specific microbial signature, Liu et al. [ ] utilized the same TCGA bladder tumor dataset, encompassing 400 MIBC patients, to explore the correlation between microbe abundance and the expression of genes associated with epithelial-mesenchymal transition (EMT) and ECM. Their investigation revealed associations between various microbes, such as E. coli , butyrate-producing bacterium SM4/1, and a species of Oscillatoria , with the expression of classical EMT-associated genes, including E-cadherin, vimentin, SNAI2, SNAI3 , and TWIST1 . Moreover, they observed that the expression levels of all above-mentioned microbes correlated with those of 275 hallmark ECM proteins.
3.3
Gut microbiome
3.3.1
Gut microbiome and bladder cancer
Recent research has revealed that the gut microbiota produces a variety of metabolites, including short-chain fatty acids (SCFAs), secondary bile acids, and polyamines, which have various effects on the development and growth of tumor [ ]. As gut microbiota (GM) exerts not only local but also long-distance effects on the host, gut microbial signaling in bladder cancer is an important aspect to evaluate.
He et al. [ ] conducted a study comparing the gut microbiota composition of BCa patients and healthy individuals. The findings highlighted a notable reduction in beneficial bacterial groups such as Clostridium cluster XI and Prevotella in BCa patients. Additionally, the concentration of butyric acid, an essential SCFA produced by these microbes, was markedly lower in the BCa group. This reduction was linked to inadequate fruit intake, which was significantly lower in patients compared to controls.
Similarly, Qin et al. [ ] performed an integrated analysis of the GM and metabolome in BCa patients and healthy controls. They discovered significant dysregulation of probiotic genera such as Bifidobacterium and Lactobacillus in BCa patients. Their study also revealed a decrease in SCFAs, specifically butyric acid, highlighting a disrupted gut microbiome composition. The altered bacterial populations were further linked to abnormal metabolic profiles, indicating a direct relationship between microbial dysbiosis and tumor progression.
Both studies underscore the importance of gut barrier integrity in cancer progression. He et al. [ ] found that lipopolysaccharide (LPS) and D-lactic acid levels were elevated in BCa patients, indicative of compromised intestinal permeability. This breakdown in the gut barrier allows harmful bacterial components to enter the bloodstream, promoting systemic inflammation and potentially contributing to tumor development and progression.
Building on these findings, Bukavina et al . [ ] evaluated GM composition in predicting the response to neoadjuvant chemotherapy (NAC) among patients with MIBC. They found that patients with a higher abundance of Bacteroides experienced a poorer response to NAC, while Akkermansia and Clostridia correlated positively with increased levels of isobutyric acid, a beneficial SCFA. Although no direct correlation was found between SCFA concentrations and chemotherapy response, the study demonstrated that machine learning models based on GM composition provided more accurate predictions for NAC response than traditional clinical factors alone.
Further evaluation of the stool microbiome in patients with MIBC undergoing neoadjuvant immune checkpoint inhibitors (ICIs) therapy, as explored by Pederzoli et al. [ ] , revealed an enrichment of the genus Sutterella in ICI responders, whereas the species Ruminococcus bromii was more prevalent in nonresponders. Together, these findings underscore the potential role of the gut microbiome in shaping treatment outcomes and highlight the future of personalized microbiome-based strategies to enhance therapeutic efficacy in BCa.
The primary research results related to microbiome and BCa can be found in Table 1 and Fig. 1 .