Pancreatic neuroendocrine tumors are rare tumors that present many imaging challenges, from detecting small functional tumors to fully staging large nonfunctioning tumors, including identifying all sites of metastatic disease, particularly nodal and hepatic, and depicting vascular involvement. The correct choice of imaging modality requires knowledge of the tumor type (eg, gastrinoma versus insulinoma), and also the histology (well vs poorly differentiated). Evolving techniques in computed tomography (CT), MRI, endoscopic ultrasonography, and nuclear medicine, such as dual-energy CT, diffusion-weighted MRI, liver-specific magnetic resonance contrast agents, and new nuclear medicine agents, offer new ways to visualize, and ultimately manage, these tumors.
Key points
- •
Knowledge of the type of functional tumor (eg, gastrinoma versus insulinoma) is important in choosing appropriate imaging strategies to identify primary lesions and their metastases.
- •
Fluorodeoxyglucose PET/computed tomography (CT) has poor sensitivity for well-differentiated pancreatic neuroendocrine tumors but good sensitivity for poorly differentiated types, and therefore has a complementary role with octreotide scanning.
- •
To optimize treatment planning, it is important to inspect all potential sites of disease, particularly with regard to the extent of liver and nodal involvement, and potential distant metastases to lung and bone.
- •
Metastatic adenopathy can show enhancement similar to adjacent vasculature structures and can be difficult to detect on CT. MRI, particularly diffusion-weighted imaging, and nuclear medicine studies can be helpful.
Introduction
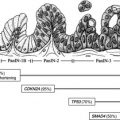
Pancreatic neuroendocrine tumors (PNETs) account for only 3% of pancreatic malignancies, but have an increasing incidence, currently 0.3 to 0.4 per 100,000. They are notably more common in multiple endocrine neoplasia type I (MEN-I), von Hippel-Lindau syndrome, neurofibromatosis type I, and tuberous sclerosis. PNETs can be divided into 2 groups: functional tumors, usually subcentimeter, that manifest because of the symptoms caused by the hormones they produce; and nonfunctional tumors, usually several centimeters, that manifest secondary to mass effect.
State-of-the-art imaging plays a central role in the identification, diagnosis, and staging of PNETs. Computed tomography (CT) and MRI are often used initially to detect and stage these lesions, and evolving nuclear medicine techniques provide improved specificity and whole-body assessments for distant disease. A multimodality approach may be necessary to identify potentially very small primary tumors and to identify all sites of metastatic disease to optimize treatment planning.
Introduction
Pancreatic neuroendocrine tumors (PNETs) account for only 3% of pancreatic malignancies, but have an increasing incidence, currently 0.3 to 0.4 per 100,000. They are notably more common in multiple endocrine neoplasia type I (MEN-I), von Hippel-Lindau syndrome, neurofibromatosis type I, and tuberous sclerosis. PNETs can be divided into 2 groups: functional tumors, usually subcentimeter, that manifest because of the symptoms caused by the hormones they produce; and nonfunctional tumors, usually several centimeters, that manifest secondary to mass effect.
State-of-the-art imaging plays a central role in the identification, diagnosis, and staging of PNETs. Computed tomography (CT) and MRI are often used initially to detect and stage these lesions, and evolving nuclear medicine techniques provide improved specificity and whole-body assessments for distant disease. A multimodality approach may be necessary to identify potentially very small primary tumors and to identify all sites of metastatic disease to optimize treatment planning.
Imaging techniques
Computed Tomography
CT is often used for the initial evaluation of patients with abdominal pain or to identify suspected small functional PNETs because of its speed, resolution, and robustness. Recent advances allow detailed multiplanar reconstructions, and new low-kilovoltage imaging or multispectral imaging may improve the conspicuity of PNETs.
A typical abdominal multidetector CT examination for PNET is multiphasic ( Figs. 1 and 2 ). Unenhanced images may be obtained to help identify calcifications or hemorrhage. At our institution, patients are then imaged following injection of iodinated intravenous contrast at 4 to 5 mL per second for an injection duration of approximately 30 seconds, with abdominal imaging obtained first at 40 to 45 seconds after the start of contrast injection for the late arterial phase of enhancement, and then 60 to 70 seconds after the start of contrast injection for the portal venous phase (see Figs. 1 and 2 ). Images are created at a slice thickness of 2 to 3 mm for diagnostic review, and at 0.625 mm for creating coronal and sagittal multiplanar reconstructions to facilitate problem solving. CT was reported in a 2009 consensus statement to have a mean sensitivity of 73% and specificity of 96%. Sensitivities for small functional tumors, such as insulinomas, vary by phase: approximately 83% to 88% for the arterial phase versus 11% to 76% for portal venous phase imaging, although a small study of 13 patients evaluating the late arterial phase (pancreatic parenchymal phase) showed a sensitivity of 100% for that phase. Detection is optimized by close evaluation of all phases.
New multispectral CT may improve detection of subtle differences in enhancement between lesions and background. Conventional multidetector CT uses a single polychromatic energy beam (eg, 120 kV peak [kVp]). Multispectral imaging uses either 2 polychromatic beams of high and low strengths (eg, 80 kVp and 140 kVp) or a dual-layer detector that can discriminate between photons of high and low energy. Multispectral imaging uses these additional data to mathematically create new types of images ( Figs. 3 and 4 ), two of the most common being monochromatic energy images (the equivalent of theoretically being scanned at a discrete single energy level), and material density or material decomposition images (semiquantitative images based on decomposing image data into representative amounts of just 2 or more materials to mimic the actual behavior at each image voxel). The precise makeup of material decomposition images varies between vendors.
Low-energy monochromatic images (ie, 40–50 keV) improve the conspicuity of contrast enhancement. A small study investigating the detection of insulinoma compared 16 patients scanned with conventional dual-phase multidetector CT with 23 patients scanned with rapid switching single-source dual-energy dual-phase multidetector CT. A sensitivity of 95.7% was obtained when a combination of low-energy monochromatic energy images and iodine (minus water) material decomposition images were used, compared with a sensitivity of 68.8% for conventional dual-phase multidetector CT.
Another related approach has been the use of a low polychromatic kVp (ie, 80 kVp) technique, which improved contrast to noise results compared with conventional 140-kVp imaging for overall pancreatic tumor imaging but did not statistically improve tumor detection. To our knowledge, no assessment has been published of this technique for PNETs.
MRI
MRI offers several advantages for the imaging of PNETs, including multiple different sequences that provide opportunities to differentiate tumor from normal pancreas. A typical abdominal protocol at our institution includes fat-suppressed T2-weighted; fat-suppressed precontrast and post-contrast dynamically obtained T1-weighted, in-phase and out-of-phase T1-weighted images; diffusion-weighted images with multiple b values and apparent diffusion coefficient (ADC) maps; and fat-suppressed true fast imaging with steady-state precession (FISP) or fast imaging employing steady-state acquisition (FIESTA) images ( Fig. 5 ). An effective slice thickness of 3 to 6 mm is used to minimize volume averaging artifact and improve sensitivity. The overall sensitivity of MRI published in a consensus report was 93%, with specificity of 88%. Limitations of MRI include greater frequency of motion-related artifacts and a longer imaging time compared with CT. Benefits include good sensitivity even in the absence of administration of an intravenous contrast agent (making it a useful alternative for patients with renal impairment or allergy to iodine-based CT contrast agents) and the absence of ionizing radiation, which is of particular concern in young patients who may require surveillance. Recent studies of advances in diffusion-weighted imaging suggest potential for improving detection, differentiating accessory spleen from small islet cell tumors, differentiating near-solid serous cystadenomas from neuroendocrine tumors, and potentially in evaluating tumor grade. MRI also offers potential advantages compared with CT in the detection of liver metastases, which is discussed later.
Nuclear Medicine
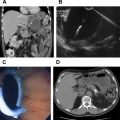
The primary nuclear medicine imaging tool for PNET is somatostatin receptor scintigraphy performed with a radiolabeled somatostatin analogue. Somatostatin is a peptide hormone for which 5 types of receptors have been identified. Octreotide, a somatostatin analogue, binds to receptors type 2 and 5. Octreotide is typically labeled with indium-111, administered intravenously, and patients are then imaged 4 hours and 24 hours after tracer administration using both planar imaging and single-photon emission CT (SPECT). At our institution, and in many centers, a CT study is performed concurrently with SPECT on a dedicated hybrid SPECT/CT camera, allowing more precise anatomic localization of octreotide uptake ( Figs. 6–8 ). Overall sensitivity for 111 In-octreotide for PNET is approximately 70% to 90% but varies with tumor type and diminishes particularly for subcentimeter lesions. Sensitivity for insulinoma, which typically expresses receptor type 3, is notably limited ( Fig. 9 ), at 50% to 70%.
Although fluorodeoxyglucose (FDG)-PET/CT would offer potentially greater precision, PNET’s don’t typically demonstrate sufficient uptake unless they are poorly differentiated. FDG-PET/CT is therefore used as a complementary technique ( Fig. 10 ) to SPECT/CT octreotide, which shows poor uptake in poorly differentiated tumors. New PET/CT agents that have been developed include gallium labeled somatostatin analogs such as DOTA-tyrosine-3-octreotide (DOTA-TOC), which has a higher affinity for somatostatin receptor type 2, and DOTA-1-NaI-octreotide (DOTA-NOC), which demonstrates a higher affinity for subtypes 2, 3, and 5 ( Fig. 11 ). Although these PET somatostatin radiotracers have faced many regulator barriers in the United States, the somatostatin analogue, DOTA-octreotate (DOTATATE, GaTate), has recently been given orphan drug status by the US Food and Drug Administration (FDA). Somatostatin receptor imaging using positron-emitting isotopes is attractive because of the improved spatial resolution of PET imaging compared with SPECT. A recent meta-analysis of 22 studies of the broad category of somatostatin receptor PET/CT, including more than 2100 patients, showed a sensitivity of 93% and specificity of 95%. The radiation dose to the patient is often lower with PET agents compared with conventional 111 In-labeled radiotracers. Although Ga-68 has been used extensively, other radioisotopes are under investigation for imaging of neuroendocrine tumors, including Cu-64 and F-18. Using the approach of theranostics, a therapeutic radioisotope (such as Y-90 or Lu-177) can be paired to the somatostatin-binding pharmaceuticals to deliver a high-dose radioisotope therapy to eligible patients, and trials are currently underway in the United States to achieve regulatory approval. In addition, metabolic pathways are being explored as a means to image PNET tumors, including PET imaging with amino acid precursors such as F-18 dihydroxyphenylalanine (DOPA), and C-11–labeled hydroxytryptophan (5-HTP).
Endoscopic Ultrasonography
Endoscopic ultrasonography (EUS) reportedly has a mean detection rate for neuroendocrine tumors of 90%. EUS-guided fine-needle aspiration (FNA) has been reported to result in overall diagnostic accuracy of 90.1%. Endoscopic ultrasonography ( Fig. 12 ) is particularly helpful for gastrinomas because many are located within bowel, a region that is poorly evaluated by both CT and MRI. The primary limitations of EUS are that it depends on the skill and experience of the operator, requires sedation, and the technique is invasive.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree