The last 30 years has seen a revolution in melanoma. Fundamental elements of the surgical, adjuvant medical, and systemic therapy for the disease have been significantly altered toward improved management and better outcomes. The intent of this article is to reflect on past efforts and research in melanoma and the current landscape of treatment of melanoma. The authors also hope to capture the excitement currently rippling through the field and the hope for a cure. The intent of treatment of advanced melanoma, which was once considered incurable, has changed from palliative to potentially curative.
Key points
- •
The last 30 years has seen a revolution in melanoma.
- •
Fundamental elements of the surgical, adjuvant medical, and systemic therapy for the disease have been significantly altered toward improved management and better outcomes.
- •
The intent of treatment of advanced melanoma, which was once considered incurable, has changed from palliative to potentially curative.
If I have seen a little further, it is by standing on the shoulders of giants.
Introduction
In 2014, an estimated 76,100 patients will develop new primary melanoma in the United States, and 9710 will die of this disease. In the United States, melanoma accounts for less than 5% of all skin cancers but is the leading cause of skin cancer mortality. The annual rate of increase of melanoma incidence is currently estimated at 3% compared with 6% in the 1970s to 1980s, but the overall mortality has been relatively stable since 1990. Worldwide the incidence of melanoma continues to increase; despite advances in local and systemic therapy, mortality continues to increase, with 80% of skin cancer-related deaths attributable to melanoma. Progress in the basic molecular biology and immunology of melanoma over the past 50 years has translated into improved outcomes for patients with localized disease as well as for those with systemic disease. The distribution of the burden of disease may be seen as inversely related to the opportunities to improve outcome. Advanced disease represents a smaller fraction of cases compared to earlier operable disease, associated with higher morbidity/mortality affording us greater opportunities for improved outcomes. Outcomes for patients with deeper primary lesions show a different picture. Those patients with deeper localized American Joint Committee on Cancer (AJCC) stage IIB/IIC disease have an increased risk of relapse and death, whereas patients with microscopic regional stage IIIA disease detectable with sentinel lymph node (SLN) mapping and biopsy have an intermediate risk. Recurrent nodal disease and bulky nodal IIIB-C disease have a relapse and mortality risk that approaches 70% or more at 5 years. Treatment and outcomes for advanced melanoma have improved over the past 20 years because of rapid advances in tumor cell biology, immunology, surgical techniques, radiosurgery, and imaging that are likely to further transform the field in the decade to come. This review reflects on the progress made in the past century and familiarizes the reader with the current state and management of melanoma.
Introduction
In 2014, an estimated 76,100 patients will develop new primary melanoma in the United States, and 9710 will die of this disease. In the United States, melanoma accounts for less than 5% of all skin cancers but is the leading cause of skin cancer mortality. The annual rate of increase of melanoma incidence is currently estimated at 3% compared with 6% in the 1970s to 1980s, but the overall mortality has been relatively stable since 1990. Worldwide the incidence of melanoma continues to increase; despite advances in local and systemic therapy, mortality continues to increase, with 80% of skin cancer-related deaths attributable to melanoma. Progress in the basic molecular biology and immunology of melanoma over the past 50 years has translated into improved outcomes for patients with localized disease as well as for those with systemic disease. The distribution of the burden of disease may be seen as inversely related to the opportunities to improve outcome. Advanced disease represents a smaller fraction of cases compared to earlier operable disease, associated with higher morbidity/mortality affording us greater opportunities for improved outcomes. Outcomes for patients with deeper primary lesions show a different picture. Those patients with deeper localized American Joint Committee on Cancer (AJCC) stage IIB/IIC disease have an increased risk of relapse and death, whereas patients with microscopic regional stage IIIA disease detectable with sentinel lymph node (SLN) mapping and biopsy have an intermediate risk. Recurrent nodal disease and bulky nodal IIIB-C disease have a relapse and mortality risk that approaches 70% or more at 5 years. Treatment and outcomes for advanced melanoma have improved over the past 20 years because of rapid advances in tumor cell biology, immunology, surgical techniques, radiosurgery, and imaging that are likely to further transform the field in the decade to come. This review reflects on the progress made in the past century and familiarizes the reader with the current state and management of melanoma.
Historical perspectives
Described in antiquity as a fatal black tumor , the term derived from Greek ( melas “dark” and oma “tumor”) was coined by Dr Robert Carswell in 1838. References to this fatal black tumor can be found in the writings of the Greek physician Hippocrates in the fifth century bc , whereas those of Rufus of Ephesus emanate from the first century ad . Hunter is credited with the first resection of melanoma in 1787. Renè Laennec described melanoma as a disease entity and coined the term melanose to describe the tumor in 1804. Dr William Norris noted the heterogeneous appearance of the tumor and its propensity to metastasize in 1820 and first noted the heritable nature of melanoma and familial atypical multiple melanoma. In further publications, he observed that most of his patients had fair skin with light colored hair and the futility of surgery and medical therapy in the setting of distant metastases. Thomas Fawdington described one of the first cases of uveal melanoma and despaired at the lack of knowledge of therapies for this “insidious” process in 1820. In 1844, British surgeon Samuel Cooper recognized the benefit of the early removal of tumors and the untreatable nature of advanced disease.
Pathogenesis
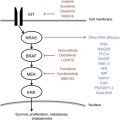
Melanocytes in the epidermis of the skin produce the pigment melanin, which occurs in several forms that variably protect the skin from UV radiation. Most melanoma is sporadic. Environmental insults followed by proto-oncogene activation coupled with the suppression of tumor suppressor genes and defects in the DNA repair mechanism further exacerbated by the inability of the immune system to contain these insults result in melanoma. William Norris presciently observed the hereditary nature of melanoma and light hair and complexion associated with melanoma in 1857. He proposed that nevi and environmental exposures predispose to melanoma, observations that were validated in the discovery of the Familial Atypical Multiple Mole and Melanoma (FAMM) syndrome and the sporadic dysplastic nevus syndrome. The connection between UV radiation exposure and increased risk of melanoma in the Australian Caucasian population was described by Henry Lancaster in 1956 whose later work demonstrated the importance of skin characteristics in the cause of melanoma. These observations gave impetus to efforts to understand the genetics of melanoma. The discovery of the melanocortin receptor 1 (MC1R) on skin/hair phenotype and its highly polymorphic nature helped make the association between pale skin/fair hair with poor tanning response (English/Celtic ancestry) and melanoma. Approximately 40% of familial melanomas were attributed to a heritable germline mutation in the cyclin-dependent kinase (CDK) gene CDKN2A. Defects in CDK4, xeroderma pigmentosum, and MC1R genes have been implicated in familial melanomas. The discovery of the role of the Ras oncogene family in the 1980s and their effects on downstream signaling were the first steps toward the identification of driver mutations in melanoma. NRAS was first identified in a melanoma cell line in 1984. The identification of the mitogen-activated protein kinase (MAPK)/ERK and PI3K/Akt pathways of melanoma tumorigenesis followed. The mutations termed rapidly accelerated fibrosarcoma (RAF) were initially identified in Ewing sarcoma. Systematic genetic typing identified the V600E variant to be frequent in cutaneous melanoma in 2002. This mutation and its constitutive activation of the MAPK pathway have become the target of multiple pharmaceutical trials of small molecule inhibitors resulting in several new therapies approved by the Food and Drug Administration (FDA). The evaluation of other histologic subtypes led to the discovery of cKIT in acral and mucosal melanomas. Uveal melanomas exhibit driver mutations in GNAQ, GNA11, and BAP1 with a low incidence of BRAF. The differing pattern of driver mutations in different histologic subtypes of melanoma and the numeric burden of mutations in different melanoma cell lines from single tumors and in tumor samples ex vivo reflect the genetic heterogeneity of melanoma and are likely to have profound implications for the molecular and immunologic therapy for melanoma.
Risk factors
Melanoma is a disease that afflicts Caucasian Americans 20 times more commonly than African Americans. The lifetime risk of melanoma is approximately 2.0% (1 in 50) for Caucasians, 0.1% (1 in 1000) for African Americans, and 0.5% (1 in 200) for Hispanics. The risk of melanoma increases with age. The average age at incidence is 61 years; but it is not uncommon among those younger than 30 years, especially young women. Men have a higher lifetime risk than women. Previous history of melanoma is associated with an approximately 7% chance of developing a second primary melanoma. Exposure to UV radiation is the predominant environmental risk factor leading to melanoma. Cumulative solar exposure and sunburn events (UV-B) are both suspected as causative. UV-A exposure from tanning beds has been implicated in the risk and incidence of melanoma, particularly among women younger than 35 years. This finding has led to the labeling of sun beds/lamps as human carcinogens by multiple health organizations, including the National Institutes of Health (NIH). Most melanomas arise as new lesions in the skin, although a significant fraction (25%–40%) seems to arise from preexisting nevi. The latter type, clinically described as atypical and pathologically identified as dysplastic nevi along with congenital nevi, has long been considered a risk marker and nonobligate precursor warranting surveillance. Giant congenital nevi (>20 cm) carry an increased risk of melanoma and are excised when possible. Family history of melanoma increases an individual’s risk of melanoma up to 8 fold. Population studies have shown an increased incidence of skin cancers, including melanoma, in patients with chronic lymphocytic leukemia. BRCA2 mutation carriers are noted to have a 2.58 times greater risk than noncarriers of developing melanoma.
Diagnosis
Patients may present with an unusual, new, or changing skin lesion to their primary care physician or dermatologist. Melanoma can present as amelanotic flesh-colored or nodular lesions. The ABCDEs of melanoma guide the decision to biopsy. The American Academy of Dermatology recommends an excisional biopsy with narrow margins as the preferred biopsy technique over shave and incisional biopsy. A deeper saucerization biopsy is acceptable, and incisional biopsy is appropriate for lesions suspicious for melanoma that are not suitable for excisional biopsy. If a punch biopsy is performed, it should be deep enough to encompass the base of the lesion. The stains traditionally used to identify melanoma include a combination of S100B (in some centers, this has been replaced with a pooled mixture of antibodies to Melan-A/MART1), HMB-45, and tyrosinase. Newer targets for IHC assessment include SOX10 and Mitf. Growth phase (radial vs vertical), morphotype (nodular or superficial spreading) mitotic rate, ulceration, and presence of tumor regression and tumor infiltration by lymphocytes are conventionally reported for their prognostic implications. Melanoma can be categorized morphologically and anatomically into the uveal, cutaneous, mucosal, and acral types, which have differing molecular patterns of driver oncogenes. Invasive cutaneous melanomas have historically been subdivided based on growth patterns into superficial spreading (most common), nodular (next most common), and acral lentiginous mucosal and lentigo maligna morphotypes, which have differing biologic behavior, prognosis, and molecular driver gene patterns.
Staging
The foundation for the current staging system was laid by the pioneering work of Wallace Clark and Alexander Breslow. In 1966, Clark proposed a system that derived from the assessment of the tissue level of invasion subsequently termed Clark level to assist in the pathologic assessment of the prognosis of melanoma. Invasion of the layers of skin reflect prognosis (Clark levels I–V representing the junction, upper papillary, full papillary, reticular, and subcutaneous zones of the skin) with decreasing survival rates associated with increased level of invasion. In 1970, Breslow observed that prognosis was affected by tumor thickness and worsened with increasing thickness measured from the granular layer of the epidermis. These systems were incorporated into clinical trial structure building the framework for surgical and medical management and serving as a common language for physicians to communicate and compare patients in clinical experiences. The AJCC Melanoma Staging Committee has periodically reassessed the staging of melanoma for prognostic assessment and last revised the staging system in 2009 (seventh edition), which was put it into practice after a period of commentary in 2010. The committee based its actions on a multivariate analysis of approximately 38,000 patients (7972 with stage IV disease) from North America, Europe, and Australia to revise/clarify the TNM classifications and overall stage grouping criteria. It incorporated key prognostic features, including Breslow thickness, ulceration, mitotic rate for thin melanomas (replaced Clark level), involvement of lymph nodes (LN) including manifestations of lymphatic spread (satellite lesions, in-transit disease), and the presence of distant metastatic disease (lung vs other). The system also integrated prognostic data for survival and risk of relapse within each stage. The staging system reflects excellent long-term survival for AJCC stage I and II melanoma, approaching 90% and 80%, respectively, at 20 years, whereas stages IIB and greater have an increased risk of relapse and death, with stage IIIB-C approaching 70% or greater relapse/mortality at 5 years.
Prognosis
The prognosis at diagnosis is largely defined by the stage of disease. Staging incorporates clinical and pathologic features and determines diagnostic and therapeutic pathways for a given individual. The extent of disease including the presence or absence of LN involvement and any distant metastases are the most important prognostic factors. The staging system also incorporates other important prognostic microstaging factors of the primary, such as Breslow thickness, presence or absence of ulceration, and mitotic rate of the primary tumor. Older patients have a worse prognosis regardless of the stage of disease compared with younger patients. Among the morphotypes of melanoma, nodular melanoma by virtue of its predominant vertical growth phase and more frequent presence of ulceration carries a worse prognosis. Acral lentiginous melanomas are more difficult to detect (inconspicuous location) and are generally first detected at more advanced stages. Lentigo maligna melanomas are generally often of longer gestation and may be detected and operated at lesser depths of invasion. Anatomically, extremity melanomas have better outcomes than truncal or head/neck melanomas. Women tend to do better than men. Ulceration serves as a marker for aggressive tumor biology and propensity to metastasize. In thin melanomas, a mitotic rate of 1 or more serves as an indication of higher risk and, therefore, warrants SLN evaluation. NRAS and BRAF mutations are also associated with differing patterns of disease aggressiveness. NRAS is associated with thicker primaries and higher mitotic rates. These markers seem to correlate with aggressive tumor biology reflecting a propensity for distant invasion.
Surgical treatment
Primary Tumor
In 1857, William Norris first recognized the importance of local disease control and advocated for a wide excision of the primary tumor with surrounding unaffected tissue to prevent local recurrence. This recommendation formed the basis of the subsequent policy for wide local excision (WLE), which is still the standard of practice today. Its scientific basis can be found in the concept that melanoma forms discontinuous nests of tumor cells in the dermal lymphatics adjacent to the primary tumor. William Handley proposed the removal of 2 in (5 cm) of surrounding tissue down to the level of muscle fascia for local control, and margins of 5 cm were accepted until the 1990s. This hypothesis was tested in multiple large trials, which looked at the impact of larger margins on overall survival (OS), local recurrence, and disease-free survival (DFS). No benefit was associated with a 3-, 4-, or 5-cm margin in terms of local recurrence, DFS, or OS. Thomas and colleagues reported the inadequacy of 1-cm compared with 3-cm WLE margins. A 1-cm WLE margin arm demonstrated an increased risk of locoregional recurrence, although the OS was similar. Data from these and other trials were summarized in a meta-analysis by Haigh and colleagues showing 1 cm margins adequate for primary melanomas of 1 mm Breslow thickness but 2-cm margins preferable for 1 to 2 mm thickness and 2-cm margins recommended for greater than 2 mm. Margins should always be negative at the time of the final pathology evaluation. Mohs micrographic surgery (MMS) is inadequately evaluated. Generally, it has been done by dermatologists who have not had facility with sentinel node biopsy, so has left patients often with margins that are hard to assess in terms of their en bloc pathologic status and lacking in the regional sentinel node assessment that has, since 2000, been recommended by the AJCC and other melanoma expert groups. The Mohs approach for cutaneous melanoma in general has been discouraged given the concern that this surgical technique ignores the biology of melanoma and the prognostic significance of discontinuous lymphatic spread and is done without the capacity to verify adequate margins in that the successive layers of skin taken in this procedure are not subjected to peer-reviewed pathologic assessment in the manner that formal wide-excision specimens conventionally may be. The fact that Mohs surgery is performed in a relatively limited number of isolated silos suggests that the lack of prospective clinical trials comparing MMS with WLE to provide more rigorous evidence-based analysis of this open question is a problem that will not soon be rectified.
Lymphadenectomy
Early on, physicians realized the aggressive nature of melanoma and its propensity for lymphatic and hematogenous metastasis and the futility of aggressive locoregional surgery once it had spread to distant sites. Early surgery with curative intent was emphasized with the goal of local-regional control. Based on the observation that melanoma initially spreads to regional LNs, Herbert Snow proposed that elective lymphadenectomy (ELND) should be included with WLE to obtain cure (1892). His hypothesis and the work of Dr William Handley (1908) reflected a stochastic model of tumor dissemination in which LNs served as a launching pad for distant metastases. Multiple prospective randomized clinical trials from 1972 onward showed no survival benefit from ELND, although the World Health Organization (WHO) trial 13 evaluating immediate LND (ILND) versus delayed LND in 240 patients with a more than 1.5-mm thick truncal melanoma suggested benefits for patients with occult microscopic LN metastases at the time of ILND. The more recent understanding of prognostic factors, such as Breslow thickness, ulceration, mitotic index, and their clinical application alongside technical advances in SLN evaluation, put this controversy to rest.
LN Evaluation
ELND for patients with truncal and head and neck region melanomas proved to be problematic given the ambiguous nature of lymphatic drainage associated with these regions. This technical issue spurred research into technologies to identify lymphatic drainage patterns; in 1977, Morton and colleagues showed that dye and radioactive tracers injected into skin around a melanoma would allow the reliable identification of the draining LNs and basins associated with truncal dermatomes. This novel technique, lymphoscintigraphy, allowed the development of the hypothesis that the SLN could guide prognostic assessment and surgery as well as other therapies for melanoma. This hypothesis postulated that a unique LN is the first to receive lymphatic drainage from a tumor site and, therefore, should also be the first for melanoma metastases. The use of vital blue dye allowed Morton and his colleagues to demonstrate that the procedure accurately identifies and allows selective biopsy of the SLN for the evaluation of potential involvement by tumor metastases. The use of blue dye along with radiotracer coupled with the portability of radiotracer detectors vastly increased the success rate of SLN biopsy. Retrospective analysis by Gershenwald and colleagues demonstrated that SLN status was the most significant prognostic factor for DFS and disease-specific survival in univariate and multiple covariate analyses. They also concluded that the SLN status should guide decisions regarding the pursuit of complete LND (CLND) in those patients (∼20%) whereby the SLN was positive. There is general consensus that SLN biopsy is justified for intermediate-thickness melanoma of more than 1 mm Breslow thickness, given that approximately 20% of these patients will have occult LN involvement. Thinner melanomas have a lower risk of regional and distant metastases. Decisions regarding SLN biopsy among patients with thin melanomas (≤1 mm) are reasonably guided by the presence of adverse prognostic factors: a mitotic rate of 1 or more per square millimeter or the presence of ulceration, tumor lymphocyte infiltration, or a deep margin positive in the original biopsy. Patients with thicker melanomas of 4 mm or more should also undergo SLN evaluation given the prognostic value of SLN involvement and the dependence of adjuvant therapy decision making on accurate staging at the LN station. Also, some patients may be cured with sentinel lymphadenectomy (SLND), especially in the setting of LN micrometastases. Positive SLN involvement is currently an indication for CLND based on the Multicenter Selective Lymphadenectomy Trial (MSLT-1). The implication of MSLT-1 and a subgroup analysis showing that all patients with micrometastatic disease benefit from CLND has generated debate. Even today, CLND remains a morbid procedure. A minority of patients with SLN involvement exhibits non–SLN involvement on CLND. MSLT-I data show that 88% of patients who have a single tumor-containing sentinel node will have no additional nodal metastases when the CLND specimen is examined. The SunBelt Melanoma Trial showed LN involvement to be approximately 16%. Survival benefits were seen only on subgroup analysis of MSLT-1. An argument can be made that the biology of SLN micrometastatic disease in the SLND group may differ from the observation group whereby palpable LN metastases later developed and that these two groups cannot strictly be compared. For most, the evidence that SLND yields critical prognostic information and may be associated with improved morbidity and mortality associated with bulky regional recurrence has led to the adoption of this approach. However, this debate continues; the questions surrounding the reasonable uses and therapeutic implications of the SLN are being pursued further in the MSLT-II trial that is ongoing. This study will randomize patients with positive LNs to either ILND or observation, with CLND reserved for patients with confirmed non–sentinel node metastases. It will also help answer the question of whether SLN biopsy in some patients is both diagnostic and therapeutic. The importance of the immunobiology of melanoma at the regional LN cannot be understated; studies now in progress at the authors’ center and others across the world are addressing the molecular profile of the SLN, which may guide the prognosis and future treatment of disease in a more refined manner. These studies are asking questions beyond whether there is tumor in the SLN or not, such as whether the SLN host response is adequate or dysfunctional as it now seems it may be in a significant fraction of patients. The SLN as a forum for biologic study, and as a pivot point for tumor progression, is likely to be a productive focus of studies on the immunobiology of this disease for years to come.
Medical management
Most patients present with early stages of diseases, so skin examination for early detection is a mandate for all physicians in primary care as well as those who specialize in the management of melanoma from medical as well as surgical and dermatologic disciplines. Data from Germany, where the state of Schleswig Holstein adopted a program of screening among dermatologists, and many primary care practitioners now suggests that routine full-body skin examination significantly reduces the incident thickness of melanoma and the mortality attributable to the disease by up to 50% based on time trends for melanoma incidence and mortality in the state of Schleswig Holstein compared with surrounding German states and neighboring Denmark. These results have already led to the nationwide pursuit of regular skin screening for detection of melanoma across Germany—and health care systems elsewhere in the world are considering the role of screening and secondary prevention on this basis. Although the German model used a day-long training module, simpler Internet-based training modules that require only 1 to 2 hours for primary care physicians may provide more practicable current alternatives; one such primary care educational effort coupled with recommended annual total-body skin screening has been initiated in the University of Pittsburgh Medical Center Health System whereby incident melanoma thickness and melanoma mortality will be followed closely over the next several years. These measures, together with evidence that appropriate surgical management cures most patients with stage IA-IB melanoma, and many with stage IIA disease, are rapidly evolving at this time. The overall 5-year survival rates for early stage disease (AJCC IA-IIA) exceed 80%. The pattern of relapse for operable early melanoma suggests that data for such patients should be judged at longer horizons. The insidious nature of some melanoma relapses and the later distribution of relapses from early stage disease make the issues of surveillance of relapse particularly important. There are wide variations in the recommended frequency, and the utilization of radiologic and biomarker studies across the world. These lie beyond the scope of this overview, except to note that the economic and patient anxiety and overdiagnosis toll of surveillance have not always been factored into recommendations (eg, S3 German, Italian, and other high-intensity programs as compared with Australian, Dutch, British, and more minimalist recommendations). Sadly, there is little rigorous evidence to support the application of interval radiologic or biomarker studies in the follow-up of patients with melanoma. Closer interval follow-ups using imaging and blood testing for biomarkers, such as S1000B, which have been favored in Europe, have not been demonstrated to translate into improved survival outcomes when compared with clinical assessment at wider intervals. The data that are available generally do not account for the enormous fiscal costs and potential for patient and societal harms of the imaging assessments proposed. Because therapies for advanced melanoma are improving rapidly, the data on surveillance for earlier cohorts of patients will not be applicable to the present and future in which therapies may have increased potential for cure of the disease in the adjuvant as well as the metastatic settings. The need to develop evidence-based approaches to the surveillance of low-, intermediate-, and high-risk patients (stage IA-B, IIA-IIIA, and IIIB-C and other resectable disease) is pressing. Evidence that would guide us in regard to which patients are at risk of late relapse is critical to determine which patients are reasonable to consider for surveillance and, ultimately, for adjuvant therapy. The role of medical therapy in reducing the risk of relapse after surgery (ie, adjuvant therapy) has been compartmentalized in relation to low-, intermediate-, and high-risk populations of patients, with variable results. Research using more informative tissue assessment is ongoing to determine the efficacy of neoadjuvant therapy in melanoma. The agents in use today have fulfilled some of the criteria that Paul Ehrlich (1854–1915) articulated more than a century ago for the Magische Kugel or “magic bullet”; these will likely change our approach to surveillance for recurrence in intermediate- and high-risk operable disease, as well as inoperable disease, as therapies that can cure metastatic disease become more generally available.
Immunology and Immunotherapy in Melanoma
Burnet and colleagues coined the term immune surveillance , which implied surveillance of the host for malignant cells, which were presumed to be recognized and destroyed as they emerged. This idea was supported by the observation of spontaneous regression in human melanoma, the lymphocytic/dendritic infiltrates documented pathologically in and around primary melanoma tumors, and the increased incidence of melanoma among immunosuppressed patients. The regression of melanoma among recipients of blood transfusions whereby the blood was derived from patients with a history of melanoma regression and the presence of tumor-specific T cells and antibodies in patients as demonstrated by Morton and colleagues showed the importance of immunology in melanoma pathogenesis. Golub and Morton were able to demonstrate in vitro increased cytotoxic activity of lymphocytes derived from patients with melanoma against melanoma cell lines. Mouse models showed the feasibility and efficacy of adoptive lymphocyte transfusion. The discovery of interleukin 2 (IL-2) as a T-cell growth factor helped pave the way for in vivo studies of immune modulation and its role in advanced melanoma. These observations and studies form the bedrock of vaccine studies, high-dose IL-2 cytokine, interferon α2b (IFNα2b), anti-cytotoxic T lymphocyte-antigen 4 (CTLA4) blocking (ipilimumab, tremelimumab), and anti–programmed death 1 (PD-1) therapies that are discussed as they have revolutionized melanoma therapy (please see later discussion).
Adjuvant Treatment
The goal of adjuvant therapy is to reduce the risk of relapse for patients by treatment at a time when measurable or gross disease is undetectable following surgery. This therapy has been pursued in melanoma as in other solid tumors to target potential micrometastases after patients have been surgically rendered disease free. Given the evidence of the immunogenicity of melanoma, adjuvant therapy has been pursued with each of the successive generations of immunomodulators that have been developed over the past 50 years, beginning with bacterial immunostimulants like Bacillus Calmette-Guerin and Corynebacterium parvum . The wider availability of interferons, first as nonrecombinant crude material purchased from the Finnish Red Cross by the American Cancer Society, was followed by the industrial production of recombinant IFN by multiple pharmaceutical firms and the first systematic dose-response evaluations by multiple routes for advanced and then adjuvant settings of disease. Kirkwood and colleagues evaluated IFNα2b in a phase I-II study in patients with metastatic melanoma and other cancers. The results showed response rates (RR) comparable with single-agent chemotherapy and durable responses in one-third of patients who responded. Phase III trials commenced in the 1980s and 1990s evaluating the role of adjuvant IFNα2b in various doses and schedules for patients with resectable deep primary and regional nodal involvement by melanoma. Based on the results of a series of studies of the Eastern Cooperative Oncology Group (ECOG) beginning in 1984, the FDA, in 1995, approved high-dose IFNα2b (HDI) for the adjuvant treatment of stage IIB and stage III melanomas. This randomized multicenter phase III study showed benefits in terms of OS and DFS compared with observation. Low-dose IFNα2b and intermediate-dose IFNα2b were then evaluated in multiple French, Austrian, and European cooperative group trials: WHO Melanoma Programs trial 16; EORTC 18871, 18952, 18991 (pegylated IFNα2b); and Nordic Melanoma Cooperative Group trials whereby a relapse-free survival (RFS) benefit was consistently observed. A recent collective meta-analysis concluded that across all dosages, an RFS benefit is observed with a hazard ratio (HR) of approximately 0.83 and an OS HR of approximately 0.91. Analyses of individual trials have shown OS benefits only with HDI as reported in the E1694 and US Intergroup trial E1694, which evaluated HDI in relation to a vaccine that is now known to have had no significant effect on either RFS or OS. Debate continues regarding the effects of alternative regimens in retrospectively identified populations such as those with ulcerated primary tumors and microscopic nodal disease, whereby current trials are prospectively testing the effects of alternative regimes of Pegylated IFN (PegIFN). The FDA based the approval of HDI, originally given for 1 year, on mature 7-year data from E1684. In 2011, the FDA approved the 5-year treatment regimen of PegIFN on data from EORTC 18991 showing overall significant RFS improvement at an early median follow-up of 3.8 years. However, a subsequent more mature analysis at a 7.6-year median follow-up for the pegIFNα2b has shown a lesser RFS benefit with HR 0.87, which on Intention to Treat analysis is of marginal significance. As there has never been any evidence of an OS impact for this regimen, the status of this agent is less clear than that for the original E1684 HDI regimen. The historic observations of early improvement of outcomes with HDI led to a series of studies testing 1 month of therapy versus 1 year, or 1 month of therapy versus observation; but these now have been rigorously tested and demonstrate no evidence of a durable benefit from 1 month alone, so that this article of investigation has been closed. Flaherty and colleagues have conducted the intergroup trial S0008 testing biochemotherapy (BCT) versus HDI and have shown a significant RFS benefit of BCT over HDI for the first time in history but without any evidence of an impact on OS. These findings may not be revisited given the rapid advances in molecularly targeted therapy for the tumor with BRAF, MEK, and possibly ERK inhibitors that have entered adjuvant exploration with results pending and the advances in immunotherapy with immune checkpoint inhibitors, such as anti-CTLA4 blocking antibodies and anti–PD-1 and anti-programmed cell death 1 ligand (PD-L1). Current clinical trials evaluating the role of ipilimumab in the adjuvant setting are enrolling across the US Intergroup and likely to complete accrual by 2014. These trials will evaluate the RFS and OS impact of ipilimumab at 3 or 10 mg/kg in relation to the standard of HDI in more than 1500 patients from ECOG-ACRIN and SWOG as well as other US Cooperative Groups and the NCI-Canada and ICORG.
Neoadjuvant Treatment
Neoadjuvant therapy in multiple solid tumors (breast, bladder, esophageal, and rectal) is associated with better outcomes in terms of survival and surgical outcomes. Given the evidence of immunogenicity and the phenomenon of immune evasion with progression in melanoma, the use of immunomodulatory agents for the neoadjuvant setting has been attractive for the past decade. Phase II trials have evaluated chemotherapy in combination and with immune modulatory agents (BCT) including such agents as IL-2, IFNα2b, cisplatin, dacarbazine (DTIC), and vinblastine. As might be expected, these trials showed a higher RR but were associated with significant toxicities. Phase III studies evaluating BCT versus polychemotherapy showed no benefit in terms of RR or progression-free survival (PFS).
The use of the neoadjuvant platform to investigate mechanisms of action beyond the response of disease in this earlier setting of potential resectability was first reported from studies at the University of Pittsburgh Cancer Institute (UPCI) in 2006 by Moschos and colleagues. Patients (n = 20) received intravenous (IV) daily IFN as in the first month of the 1-year E1684 regimen, followed by surgery with the goal of evaluating both response and tissue correlates of therapy. Ten percent of patients (n = 2) demonstrated pathologic complete response (CR) and 40% (n = 8) had partial responses (PR). Tumor specimens systematically evaluated before and following therapy showed increased T-cell and dendritic cell populations with radical changes in the level of constitutively expressed STAT3 in the tumor tissue over the month of IV IFN. The same approach undertaken at UPCI with ipilimumab showed increased tumor infiltration by activated T cells with induction/potentiation of memory T cells. The change in Treg observed within the tumor showed an inverse relationship with the clinical benefit and was associated with improved PFS at 1 year. The study of the combination of ipilimumab and IFN is now ongoing in the neoadjuvant setting at UPCI to pave the way for this combination in future phase III trials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree