Surgeon(s)
Year of procedure or publication
Operative accomplishment
Pean
1879
Unsuccessful pyloric resection
Rydygier
1880
Unsuccessful pyloric resection
Billroth
1881
First successful pyloric resection (Billroth I)
Kocher
1893
Posterior gastroduodenostomy
Billroth
1885
Antrectomy after loop gastrojejunostomy (2-stage, Billroth II)
Krönlein
1887
End-to-side gastrojejunostomy
Woelfler
1881
Y-gastroenterostomy
Roux
1893
Retrocolic Y-gastrojejunostomy
Connor
1884
Unsuccessful total gastrectomy
Schlatter
1897
First successful total gastrectomy
Brigham, Richardson
1898
Three successful total gastrectomies
Hoffmeister
1908
Greater curvature gastrojejunostomy
Reichel–Polya
1911
Full length gastrojejunostomy
McNeer
1951
Radical gastrectomy, extended lymphadenectomy
Appleby
1953
Radical en bloc gastrectomy with resection of celiac artery
Hunt
1952
Pouch esophagojejunostomy reconstruction
Merendino
1955
Small bowel interposition reconstruction
Kitano
1992
Laparoscopically assisted distal gastrectomy
Azagra
1996
Laparoscopic total gastrectomy
Surgical Objectives
General objectives of operative therapy for gastric cancer are more easily compiled than successfully accomplished on a consistent basis in clinical practice [4]. They include surgical removal of tumor tissue, the provision of local and regional disease control, the optimization of curative potential, the provision of intraoperative and pathologic staging information which occasionally includes the confirmation of a gastric cancer diagnosis, the restoration of function lost or limited through a resection such as reestablishing gastrointestinal continuity after gastrectomy, and minimizing any resulting postoperative morbidity. The latter objective, for example, would include strategies to avoid splenectomy or distal pancreatectomy if possible to reduce infectious morbidity or to furbish gastric rather than esophageal anastomoses when feasible to minimize anastomotic leaks. While all listed objectives appear equally valid in a minimally invasive surgery (MIS) context, improved recovery potential and minimized morbidity obviously carry special appeal for a MIS rationale and approach to gastric cancer treatment.
Operative Intent
The intent to conduct an operation for gastric cancer can be highly variable. In most cases, a procedure is justified to cure the underlying malignancy. Curability criteria for surgical therapy depend on the underlying disease extent and biologic behavior and are set by the relatively limited scope of local and regional tumor control a resection is able to accomplish [5]. In any circumstance, a curative outcome after resection cannot be expected unless all known locoregional disease is completely removed, usually en bloc, and is generally not possible if diffuse extraregional metastatic disease does exist. Even if the complete removal of all gross disease with negative margins (R0 resection) has been performed, subsequent recurrence remains common for gastric cancers of mid-stage due to the presence of nonvisualized micrometastases at the time of operation [6]. This mechanism and the fact that previously undetected metastatic disease is identified intraoperatively are the most common reasons if a preoperative curative intent cannot be achieved [4]. Macroscopically visible residual disease and positive peritoneal cytology are virtual guarantees for symptomatic disease recurrence to develop [7]. Microscopic positive margins (R1 status) impart an increased local recurrence risk, but are in addition a surrogate for higher-risk disease and a greater failure rate in extraregional sites [8]. In addition to the curative intentions, a diagnostic component or the provision of tumor tissue for specific purposes can provide the rationale to operate on a patient with gastric cancer, specifically if the diagnosis is suspected but remains unconfirmed through endoscopic biopsy means, or if more advanced intra-abdominal disease extent is suspected but not confirmed through imaging modalities. Another common preoperative intent is the palliation of symptoms that cannot be alleviated through lesser invasive means such as endoscopy or interventional radiologic techniques [9]. Examples for this approach are obstruction symptoms not relieved through stent placement or resection needs for tumor-related bleeding not amenable to palliative radiation or interventional vascular manipulation. In this context it is important not to confuse the terms “palliative” and “noncurative”; a noncurative operative procedure is hardly ever justifiable in a patient who does not suffer from symptoms that require a specific surgical intervention, while an operation with palliative intent is primarily driven by the patient’s symptoms irrespective of whether potential curability is still given or not [10]. Therefore, preoperative intents for operative therapy for gastric cancer can exist in single or in combined form (Table 7.2). The surgeon is advised to clearly define preoperative intents, for guidance of the informed consenting process with patient and family members, for appropriate positioning of the operative step within the sequence of multidisciplinary treatment options, as well as for enabling correct interpretation of outcomes. Preoperatively clearly defined palliative or diagnostic intents for operations have a greater chance to be successfully achieved compared to procedures performed with curative intent [4].
Table 7.2
Preoperative intents to provide operative therapy for gastric cancer
Intent | Examples | Comments on requirements or conditions |
|---|---|---|
Diagnostic | Diagnostic laparoscopy | Enhances clinical staging either prior to induction therapy or at beginning of planned resection; rarely required to prove and treat suspected gastric cancer that failed endoscopic biopsy confirmation attempts |
Curative | Gastrectomy with D2 lymphadenectomy | Requires absence of extraregional metastases; all multimodality options considered; goal not achieved through R2 and most R1 resections; need for symptom control may affect timing of resection |
Palliative | Intestinal bypass for malignant peritoneal bowel obstruction, gastrojejunostomy bypass | Nonoperative or less invasive options always preferred if feasible; possible benefits to resection of tumor reported only in low tumor burden settings; although most often in noncurative setting, palliative-intent gastrectomy can result in curative procedure if disease extent is smaller than expected |
Noncurative | Gastrectomy for asymptomatic stage IV tumor | Cannot be supported or justified without other compelling intents documented |
Preemptive | Resection of gastric tumor to prevent obstruction in setting of metastatic disease | Hardly ever indicated; should not prompt a separate planned operation |
Supportive | Surgical feeding jejunostomy tube placement prior to preoperative therapy | Nonoperative or less invasive means preferred if possible |
Tissue provision | Resection of gastric cancer tissue for on-protocol vaccine generation | Hardly ever indicated; less invasive nonoperative means preferred |
Operative Therapy as Part of a Multidisciplinary Strategy
Due to the high risk for recurrence after resection of mid-stage GC, additional treatment options have been increasingly applied. In numerous phase 3 randomized controlled trials, adjuvant therapy has been demonstrated to lead to superior overall survival (OS) compared to gastrectomy alone [11]. Adjuvant therapy options with OS benefits and particular relevance to practice within the United States include postoperative chemotherapy with chemoradiation according to the Intergroup 0116 trial [12], perioperative chemotherapy analogous to the MAGIC or ACCORD07 trials [13, 14], or preoperative chemoradiation analogous to the CROSS trial [15]. Details on these multimodality treatment options exceed the scope of this chapter. As there is currently no single, evidence-based approach to multimodal GC therapy, various regimens are in use based on local centers’ expertise and preference. In general, preoperative (neoadjuvant) approaches are preferred, as tolerance to treatment is greater, delivery is more likely complete, and as clinical or pathologic response to such treatment may represent an important prognostic surrogate for disease behavior and future recurrence risk [16, 17]. Perioperative chemotherapy appears to be most useful for mid- and distal third gastric tumors, while preoperative chemoradiation may be preferred for proximal gastric or GE junction lesions. Importantly, any operation plans would have to be balanced against these important strategies, especially for curative goals, and formal multidisciplinary evaluation of appropriate treatment options prior to initiation of therapy should be mandatory. “Surgical” therapy of GC therefore includes knowledge of and support for multimodality treatment and the insight to adapt to effects of other treatments, especially regarding assessment of tumor response to preoperative therapy and delineation of an appropriate resection extent.
Preoperative Aspects
Most patients will present to the surgeon with biopsy-proven adenocarcinoma through endoscopic means. Accurate clinical staging includes computed tomography imaging and endoscopic ultrasound (EUS) evaluation. It is important to have precise documentation in regard to primary tumor location and extent prior to initiation of preoperative therapy, as responses to this treatment may render intraoperative localization attempts difficult. PET scans do not appear mandatory for GC staging, but may have a more reliable role in proximal or GE junction primaries or to guide preoperative chemotherapy on protocol; diffuse-type GCs tend to be less well imaged on PET scans [18, 19]. Resectable tumors are best approached in terms of resection extent based on their pretreatment extent and stage, irrespective of restaging findings. Even major clinical responses are often incomplete on pathologic examination, supporting this more “radical” approach [20, 21]. An exception would be the rare scenario of an unresectable tumor being rendered resectable due to a response to initial chemotherapy or radiation. The intraoperative specifics are thus best delineated preoperatively, including planned operative approach (open versus laparoscopic), placement of incision(s), resection extent, and preferred reconstruction. Staging laparoscopy is strongly recommended as an operative complement to preoperative imaging, as it is most sensitive in detecting small-volume peritoneal or visceral surface metastases [22, 23]; laparoscopic ultrasound may slightly increase metastasis detection rates [24]. In addition, peritoneal washing cytology may be considered if subsequent treatment steps are affected by positive results [25]. Timing or frequency of staging laparoscopy around preoperative therapy is being debated [26]. Patients with persisting positive washing cytology findings invariably have poor OS outlook, while those with positive peritoneal cytology status that turned negative have shown longer survival [7].
Standards for Curative Mid-Stage Gastric Cancer Operative Therapy
Technical Aspects of Resection
State-of-the-art curative-intent gastrectomy requires R0 resection and should be accompanied by an extended lymphadenectomy (D2 dissection) [27, 28]. Whether open or minimally invasive surgical (MIS) techniques are utilized appears to be of lesser consequence oncologically, as long as principles of complete local resection and regional dissection are adhered to [29–32]. The following operative components are based on open gastrectomy standards, but seem to be equally relevant for a MIS approach. For early GC (T1N0), endoscopic mucosal resection (EMR) or submucosal dissection (ESD) may suffice as definitive therapy [33]; both require proper specialty skills and currently appear to be limited to few centers within the United States with appropriate technical and clinical expertise. EMR and ESD techniques will not be described in further detail within this chapter. For all more advanced stages of nonmetastatic gastric adenocarcinoma, complete locoregional resection is the central component for curative-intent therapy. In the operating room, general endotracheal anesthesia is introduced, and the patient is usually placed in a supine position for a planed open celiotomy; planned laparoscopic resection may favor different positions based on the operating surgeon’s preference. It may be helpful to consider a short repeat upper gastrointestinal endoscopy after induction of anesthesia prior to resection or later for anastomotic assessment [34]; the author has used this liberally to verify tumor location and extent and to assess the mucosal appearance of gastric or esophageal components to be used in the reconstruction or for anastomotic sites. In addition, laparoscopy should be performed now unless already done in a separate setting. In up to 20 % of cases, laparoscopic confirmation of intra-abdominal metastases will still provide the opportunity to avoid an otherwise noncurative gastrectomy in this setting.
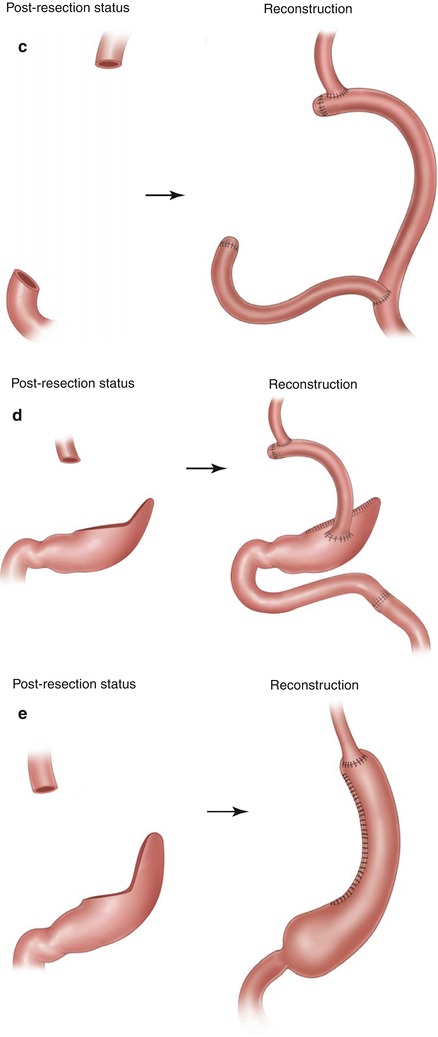
A transabdominal approach will be sufficient for most complete resections, but incision placement for open gastrectomy is not standardized and follows personal preferences. While many surgeons choose upper midline incisions, the author prefers a bilateral subcostal margin incision approach. Rarely is there benefit to a combined left thoracoabdominal incision, but for high, large gastric tumors in obese patients, this can generate much superior exposure if needed. A routine thoracoabdominal approach for GC resection is not beneficial compared to the transabdominal-only access and thus not recommended [35, 36]. With proper exposure and resectability established, the main resective objectives are R0 resection and lymphadenectomy. Total gastrectomy out of principle is not necessary; lesser extent resections, especially for distally located tumors, have shown comparable survival results, with fewer morbidity and functional challenges [37, 38]. Appropriate macro- and microscopically negative margins should be obtained as feasible at duodenal and esophageal resection sites. In challenging scenarios of advanced disease burden, it can be acceptable to leave a positive margin at these sites, as long as parameters such as serosal involvement or significant nodal burden imply a minimal curative potential. Intragastric margins of 5 cm are traditionally recommended for subtotal gastrectomy, at least for intestinal-type disease [39, 40]; diffuse-type lesions may require wider margins. A healthy tissue esophageal margin length of 2 cm seems to be sufficient for resection of Siewert type II and III lesions treated with gastrectomy [41]. The choice of gastrectomy extent (and of lymphatic dissection extent) will not only depend on location and extent of the primary tumor but also on potential reconstruction needs and options (Fig. 7.1). In general, for distal lesions a subtotal gastrectomy is adequate. For lesions in the middle third of the stomach, the decision between total or near-total gastrectomy depends on the proximal margin status and considerations for possibly safer reconstruction (gastrojejunostomy leak rates have been described as occurring half as often as those after esophagojejunostomy [42]). Proximal third lesions will essentially always require either total gastrectomy or proximal gastrectomy with a special reconstruction such as small bowel interposition [43]. Proximal gastrectomy with subsequent esophagogastrostomy is not recommended, especially after pyloroplasty, for concerns of significant reflux. Avoiding any pyloromyotomy or pyloroplasty in this setting is recommended, but does not completely preempt reflux-related problems; distal gastric emptying problems that require endoscopic or even operative management may occur in 5–15 % of cases. Lesions at the GE junction require special operative planning based on the lesions’ epicenter and, more importantly, the proximal disease extent. Siewert type I lesions require a transthoracic or transhiatal esophagectomy and should not be approached with an attempt to perform a gastrectomy [44]. Siewert type II lesions are located at the gastric cardia; these can either be approached via esophagogastrectomy with retrogastric LND analogous to type I lesions or through an extended gastrectomy as long as not more than 3 cm of distal esophageal involvement exists and proximal negative margins (of 2 cm or greater) can be obtained [45]. Siewert type III lesions are in biologic terms proximal gastric cancers, and a transabdominal approach should be fully sufficient as long as no more extensive submucosal esophageal involvement exists [44, 45]. It appears permissible to decide upon the best resection extent for proximal gastric cancers close to the GEJ intraoperatively through esophageal transection and frozen section analysis, as long as the surgeon is experienced with performing an esophagectomy in this setting and prepared to do so if necessary and as long as right gastric and gastroepiploic vasculature is initially preserved for a gastric tube reconstruction in case an esophageal resection becomes necessary.
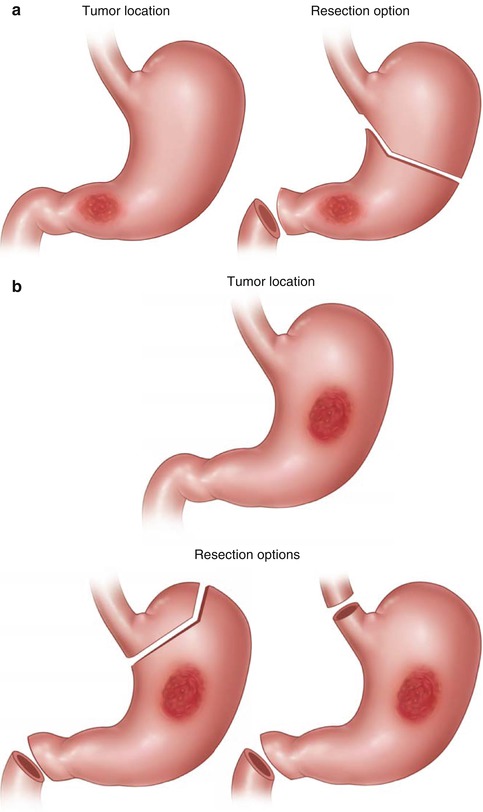



Fig. 7.1
Schematic representation of gastric resection extent based on the location of the primary adenocarcinoma. (a) Resection extent for distal third tumors; (b) resection extent options for middle third tumors; (c) resection extent options for proximal third tumors including Siewert type III lesions; (d) resection extent options for Siewert type II lesions
Total or near-total omentectomy is frequently performed en bloc with a gastrectomy for cancer and represents a good way to initiate the dissection. Omental bursectomy has been widely applied as a means to accomplish more complete resection of posterior wall lesions; it includes removal of the anterior peritoneal leaf of the mesocolon in an attempt to not enter the lesser sac and completely remove this retrogastric structure. While it appears less sensible from an oncologic standpoint, especially for transmural tumors with serosal involvement and progression risks [46, 47], it nevertheless appears to be a useful technique to identify the relevant retroperitoneal plains above the pancreas for identifying lymph nodes at hepatic and splenic arteries. Careful attention is applied to not injure the pancreas parenchyma in the process. For all gastric tumors except those close to the GE junction, the proximal duodenum is freed and prepared for transection; in this process, dissection of gastroepiploic LNs off the underlying pancreas and deep ligation and transection of gastroepiploic vessels will keep the inferior parapyloric and gastroepiploic (level 6) LNs on the specimen and will allow for easy access to the duodenum. The dissection is now carried from distal to proximal, with division of lesser omentum and mobilization of paragastric tissues at the lesser curvature up to the diaphragmatic crus. If the extended LND is to be performed en bloc, common hepatic artery LNs are now mobilized and kept with the specimen. The origin of the left gastric artery should always be identified and divided for cancer resections; splenic artery nodes are dissected away from pancreas and artery, and short gastric vessels are divided close to the spleen. The spleen can most frequently be preserved unless direct tumor involvement or a large hilar LN burden requires splenectomy. Splenic hilar LN involvement is rare for tumors not located at the fundus or proximal two thirds of the greater curvature. Even when splenic hilar dissection is desired in fundus or greater curvature primaries, spleen-preserving hilar LN dissection has been applied, since spleen preservation may have important benefits for reduced postoperative morbidity [48–51]. The proximal transection is now determined based on anticipated margin needs. This is either at the level of the distal esophagus or transgastric with preservation of the proximal stomach if feasible. In the latter scenario, the lesser curvature transection should extend close to the GE junction without narrowing the esophagogastric passage, primarily to support a complete left gastric artery LND, while more length can be preserved toward the greater curvature if possible. This then shall allow for an easier reconstruction, with a subsequent anastomosis close to the greater curvature transection site. Completion of the retroperitoneal dissection with celiac lymphadenectomy and clearance of tissues to the diaphragmatic crural tissue completes the gastrectomy. For locally advanced tumors, multivisceral resections are occasionally necessary and indicated when resulting in a R0 resection that still offers curative potential. In this situation, the surgeon ought to be prepared to perform an en bloc segmental hepatectomy, diaphragmatic resection, pancreatosplenectomy, left adrenalectomy, or colectomy as required.
Additional Aspects of Lymph Node Dissection
The propensity of gastric adenocarcinomas to involve lymph nodes (LNs) is high. Although actively debated over the past decade, lymphadenectomy at the time of curative-intent gastrectomy has shown benefits to staging accuracy and to cancer control and has thus become standard of care [28, 52]. Resection of the appropriate paragastric and of second echelon (left gastric, common hepatic, splenic, celiac artery) LNs (D2 dissection) is generally sufficient; wider dissections have not shown superior results [35]. This procedure should yield at least 15 or more LNs for the pathologic evaluation, but greater total LN counts have been associated with better survival outcomes [53–55]. A long-term survival or disease-specific control benefit to extended LN dissection (ELND) has now been demonstrated in at least two randomized controlled trials, despite a greater early morbidity and mortality in the Dutch trial after D2 dissection [28, 52, 56]. These were related to an increased rate of pancreatosplenectomy with D2 dissection [57], but this survival hazard has been superseded by a long-term overall survival benefit due to greater disease control. As discussed earlier, splenectomy and distal pancreatectomy are strongly discouraged unless deemed necessary based on tumor involvement [58, 59].
ELND can be performed en bloc with the gastrectomy as described above, or in a separate specimen. The paragastric nodes (i.e., paracardial, lesser and greater curvature, right gastric artery, and gastroepiploic artery LNs) are always best removed with the adjacent stomach portion. Since the LN group to be removed is variable based on the tumor location, a good strategy is to remove any paragastric LNs adjacent to stomach that is also to be removed. Dissection of the named artery LNs will then complete a sensible D2 dissection. If these left gastric, common hepatic, splenic, and celiac artery LNs do not appear grossly abnormal, the author has divided the left gastric artery pedicle to facilitate gastrectomy as initial step, to be followed by the retroperitoneal dissection of these structures as second step (Fig. 7.2). This allows not only for better exposure but also improved pathologic identification of relevant retrogastric LN involvement. The left gastric artery should generally be divided in cancer resections, in part for better nodal clearance; occasionally, an accessory left hepatic artery is encountered that can be preserved, as LNs can be dissected around the proximal left gastric artery, and the gastric branch can be divided after separating from the hepatic branch. In most Western patients, it is not possible to identify all LNs of interest visually during the dissection. The goal is therefore to free the relevant and named arterial vasculature of all surrounding lympho-areolar and adipose tissue, rather than obtain specific LNs or a certain total number of LNs. LN counts are determined by the pathologist and do not only reflect radicality of dissection, but also quality of the specimen pathologic examination, and other clinicopathologic factors including preoperative therapy effects and nutritional implications. A median total LN count between 20 and 30 appears to be an acceptable standard [27, 53, 54]. In some Asian centers, limiting the LND in patients with low likelihood for LN involvement is being explored, such as through laparoscopic sentinel LN biopsy for early GCs [60, 61], but these techniques are not yet accepted as proven standards.
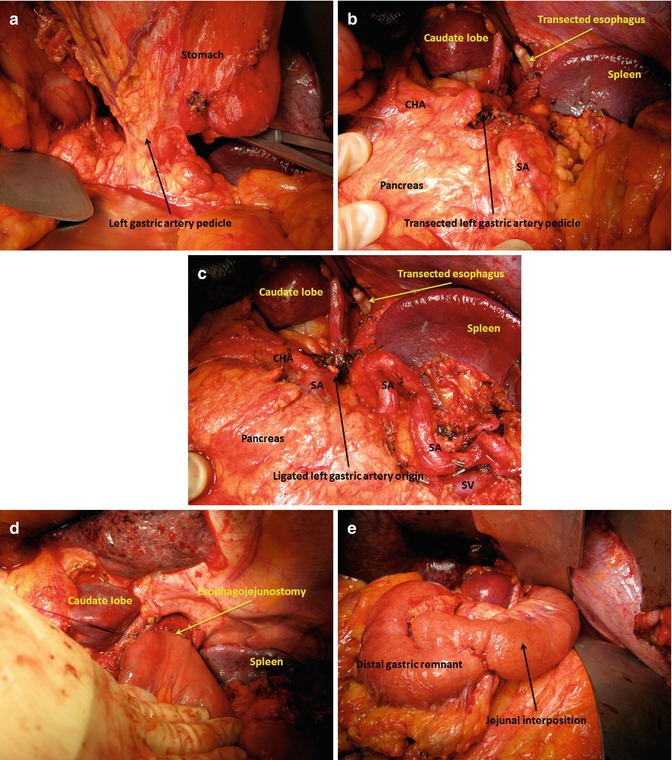

Fig. 7.2
Intraoperative images of a 2-step extended lymphadenectomy and subsequent reconstruction (a) Appearance of the left gastric artery pedicle during resection of a proximal gastric cancer; (b) Appearance after transection of the left gastric artery pedicle and proximal gastrectomy. CHA common hepatic artery, SA splenic artery; (c) Completion of retroperitoneal lymphadenectomy at celiac, hepatic, and splenic arteries. CHA common hepatic artery, SA splenic artery, SV splenic vein; (d) Completed esophagojejunostomy; (e) Completed jejunogastrostomy between small bowel (Merendino) interposition and distal remnant stomach
Technical Aspects of Reconstruction
Most gastric resections are followed by Roux-en-Y jejunal reconstruction, either as esophagojejunostomy or gastrojejunostomy (Fig. 7.3). The jejunal limb is best created with a length of around 45 cm to achieve the lowest degree of both Roux-stasis and of dumping problems postoperatively [62]. Billroth 1 and 2 reconstructions have been described after distal gastrectomy, but appear acceptable regarding appropriate oncologic dissection extent and functional outcomes only for very distally located tumors [63, 64]. A potentially challenging scenario for either reconstruction technique is that of a small proximal gastric reservoir with uncontrolled access of biliary small bowel contents and the related bile reflux risk [65, 66]. Similarly, after proximal gastrectomy, a small distal reservoir too and biliary reflux have to be avoided. A Merendino small bowel interposition between the esophagus and distal gastric reservoir and the avoidance of pyloric manipulation if possible present acceptable options, as shown in (Fig. 7.2) [43]. As a general important aspect, reconstruction preferences should not compromise the resection extent. Pouch reconstructions are rarely performed in the United States as there has been no convincing evidence of postoperative nutritional superiority; some reports describe a potential long-term quality of life benefit [67, 68].