The quality of surgical treatment is a major determinant of cancer treatment outcomes; however, controlling surgical quality is a difficult task. Surgical treatment of gastric cancers, and especially the benefits of nodal dissection, has been a topic of debate and no consensus has been reached to date. The D2 nodal dissection defined, standardized, and practiced in Japan is a technically challenging procedure but carries better locoregional disease control. This article reviews the current definition of D1, D1 plus, and D2 nodal dissections, as well as the nodal dissection technique, indications for its modification, and the learning curve.
D2 nodal dissection for the treatment of a gastric cancer was initially proposed by a Japanese surgeon, Tamaki Kajitani, in 1942 when the long-term result of surgical resection carried poor prognosis for this disease. Since then, numerous studies of lymphatic pathways have been performed and nodal stations around the stomach defined. In 1962, the first edition of the Japanese Classification of Gastric Cancer was published by the Japanese Research Society for Gastric Cancer (JRSGC), which defined the classifications and rules for the treatment of gastric cancer. In Japan, this was widely accepted and operations were standardized for the location of the primary lesion and the stage of the disease. Originally, the D2 nodal dissection, which removes all the first-tier and second-tier nodal stations, was recommended for all stages of gastric cancer. In recent years, these recommendations have been modified for early gastric cancer.
The extent of the nodal dissection for gastric adenocarcinoma has been a topic of debate for a few decades. The most recent update of the Dutch D2 – D1 trial showed improved locoregional disease control in the D2 group but failed to show improvement in overall survival. The study was criticized for underdissection or overdissection of the nodal stations as well as the high morbidity/mortality associated with the pancreatosplenectomy, which canceled out any potential survival benefit of the D2 nodal dissection. The surgical quality control and the learning curve on an unfamiliar procedure proved to be a major difficulty in a surgical trial (see the articles by Sasako and Hundahl elsewhere in this issue).
Intuitively, the groups of patients with gastric cancer who receive maximum potential benefit from the D2 nodal dissection are those with stage Ib to IIIa by the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, seventh edition. In 2004, the Japanese Gastric Cancer Association (JGCA, formerly the JRSGC) published the second edition of the gastric cancer guideline and defined the modified nodal dissections. In 2010, refinements and simplifications of the previous guidelines were put forth based on published nodal metastasis data from the National Cancer Center and Cancer Research Institute in Japan. Since then, patients with early gastric cancer (stage Ia) are offered minimally invasive gastrectomies with modified nodal dissections in Japan. This article discusses the anatomy and the exposure of the nodal basins (stations) necessary to perform D2 nodal dissection, modifications to the standard nodal dissection based on tumor location and stage, and the difficulties presented by these demanding procedures.
Definition of the nodal stations and the D1 and D2 nodal dissections
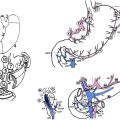
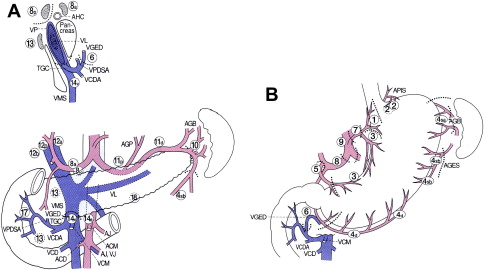
The nodal stations around the stomach are anatomically defined and numerically classified by the Japanese Classification of Gastric Carcinoma published by JGCA ( Fig. 1 , Table 1 ). Perigastric nodal stations are numbered 1 to 6 and regional nodal stations are 7 to 12. Nodal stations numbered higher than 12 are generally considered distant nodal stations and are not dissected for the standard D2 nodal dissection, except nodal station 14v (discussed later).
| No. | Definition |
|---|---|
| 1 | Right paracardial LNs, including those along the first branch of the ascending limb of the left gastric artery |
| 2 | Left paracardial LNs including those along the esophagocardiac branch of the left subphrenic artery |
| 3a | Lesser curvature LNs along the branches of the left gastric artery |
| 3b | Lesser curvature LNs along the second branch and distal part of the right gastric artery |
| 4sa | Left greater curvature LNs along the short gastric arteries (perigastric area) |
| 4sb | Left greater curvature LNs along the left gastroepiploic artery (perigastric area) |
| 4d | Right greater curvature LNs along the second branch and distal part of the right gastroepiploic artery |
| 5 | Suprapyloric LNs along the first branch and proximal part of the right gastric artery |
| 6 | Infrapyloric LNs along the first branch and proximal part of the right gastroepiploic artery down to the confluence of the right gastroepiploic vein and the anterior superior pancreatoduodenal vein |
| 7 | LNs along the trunk of the left gastric artery between its root and the origin of its ascending branch |
| 8a | Anterosuperior LNs along the common hepatic artery |
| 8p | Posterior LNs along the common hepatic artery |
| 9 | Celiac artery LNs |
| 10 | Splenic hilar LNs including those adjacent to the splenic artery distal to the pancreatic tail, and those on the roots of the short gastric arteries and along the left gastroepiploic artery proximal to its first gastric branch |
| 11p | Proximal splenic artery LNs from its origin to halfway between its origin and the pancreatic tail end |
| 11d | Distal splenic artery LNs from halfway between its origin and the pancreatic tail end to the end of the pancreatic tail |
| 12a | Hepatoduodenal ligament LNs along the proper hepatic artery, in the caudal half between the confluence of the right and left hepatic ducts and the upper border of the pancreas |
| 12b | Hepatoduodenal ligament LNs along the bile duct, in the caudal half between the confluence of the right and left hepatic ducts and the upper border of the pancreas |
| 12p | Hepatoduodenal ligament LNs along the portal vein in the caudal half between the confluence of the right and left hepatic ducts and the upper border of the pancreas |
| 13 | LNs on the posterior surface of the pancreatic head cranial to the duodenal papilla |
| 14v | LNs along the superior mesenteric vein |
| 15 | LNs along the middle colic vessels |
| 16a1 | Para-aortic LNs in the diaphragmatic aortic hiatus |
| 16a2 | Para-aortic LNs between the upper margin of the origin of the celiac artery and the lower border of the left renal vein |
| 16b1 | Para-aortic LNs between the lower border of the left renal vein and the upper border of the origin of the inferior mesenteric artery |
| 16b2 | Para-aortic LNs between the upper border of the origin of the inferior mesenteric artery and the aortic bifurcation |
| 17 | LNs on the anterior surface of the pancreatic head beneath the pancreatic sheath |
| 18 | LNs along the inferior border of the pancreatic body |
| 19 | Infradiaphragmatic LNs predominantly along the subphrenic artery |
| 20 | Paraesophageal LNs in the diaphragmatic esophageal hiatus |
| 110 | Paraesophageal LNs in the lower thorax |
| 111 | Supradiaphragmatic LNs separate from the esophagus |
| 112 | Posterior mediastinal LNs separate from the esophagus and the esophageal hiatus |
The level of the nodal dissection, known as the D number, is defined by the guidelines from JGCA. Although the classic D1 nodal dissection is defined by complete dissection of the first-tier nodal stations, which are determined by the location of the primary lesion, and is most compatible with the current concept of the D1 nodes, perigastric nodes (station 1–6) in Western literature, the current (2010) definition of D1 nodal dissection in Japan includes the left gastric artery node station (station 7) in addition to the perigastric nodal stations because of the observed high rate of metastasis in this nodal station by early gastric cancer.
The technique of D2 nodal dissection (distal and total gastrectomy)
Typically, a D2 nodal dissection starts from the greater curvature of the stomach. An omentectomy is an integral part of the D2 nodal dissection. The lesser sac is accessed by detaching the greater omentum from the transverse colon. Once it is widely separated then the greater omentum can be seen to be fused with the anterior surface of the transverse colon mesentery on the right side ( Fig. 2 ). This portion is considered right side border of the lesser sac. Access to station 6 (the infrapyloric nodal station) is gained by further separating the omentum from the transverse colon mesentery by dividing this right side border peritoneum (see Fig. 2 yellow line). Once they are completely separated then the middle colic vessels and a right accessory colic vein can be seen ( Fig. 3 ). By tracing the middle colic vessels, the anterior surface of the superior mesenteric vein (SMV) can be reached. The soft tissue covering the anterior surface of the SMV below the lower edge of the pancreas is classified as station 14v. If the tumor is located in the antrum of the stomach, then this station may be included in the resection (discussed later). The right accessory colic vein is located in the right side of the transverse colon mesentery, this joins with the right gastroepiploic vein, and the venous drainage from the pancreatic head then forms a gastrocolic trunk. This large vein then drains directly into the SMV ( Fig. 4 ). The right accessory vein is sometimes called an accessory middle colic vein; however, this article follows the JGCA and uses the term right accessory colic vein. Nodal station 6 is located proximal to the junction of the right accessory colic vein and the right gastroepiploic vein. For complete dissection of station 6, the right gastroepiploic vein should be ligated at this junction. All the soft tissues covering the anterior surface of the pancreas head are then dissected off toward the pylorus. About 5 mm to 1 cm cephalad to the right gastroepiploic vein, a right gastroepiploic artery can be seen emerging from the pancreatic head parenchyma ( Fig. 5 ). This artery is ligated at this portion and all the soft tissue are cleared from the pancreas toward the inferior wall of the duodenum, which concludes the infrapyloric portion of the dissection.
At the lesser curvature of the stomach, the lesser omentum is incised along the attachment about 1 cm from the liver. The incision extends up to the esophageal hiatus. An accessory (or replaced) left hepatic artery should be recognized in the area, if present. It can be preserved by dissecting all the surrounding soft tissues if the gastric cancer is early and has a low chance of involvement of the left gastric nodes. On the left side of a hepatoduodenal ligament, soft tissue covering the proper hepatic artery is dissected toward the common hepatic artery (station 12a). As the dissection progresses toward the common hepatic artery, the origin of the right gastric artery can be seen, and this is ligated at the origin and the suprapyloric portion of the soft tissue along this artery (station 5) is separated from the hepatoduodenal ligament and head of the pancreas toward the superior duodenal wall.
A retroperitoneal dissection can be performed without transecting the duodenum but exposure is usually better after the transection. The retroperitoneal dissection starts as a continuation of the previous proper hepatic artery node dissection. The peritoneum is divided along the superior border of the pancreas to the left and the soft tissue covering the common hepatic artery (station 8a) is dissected away. There is a distinctive plane between the nodal tissue and the pancreas parenchyma; this should be recognized and the dissection should be maintained in this plane to avoid injury to the pancreas. There are multiple small vessels present between these nodes and upper border of the pancreas and these should be recognized and coagulated before transection. The dissection plane can also be maintained just outside the perivascular nerve plexus unless gross metastatic nodes present along the artery. The left gastric vein is typically present around the common hepatic artery but may be located in front of the common hepatic artery. Injury to this vein, often referred to as the coronary vein, creates a bleeding situation that is difficult to control. Careful dissection and vessel control before division is recommended. The superior border of the dissection is the right crus of the diaphragm.
Once the origin of the left gastric artery is identified, the soft tissue surrounding the artery is dissected distally and the vessel is ligated at the origin. After dividing the left gastric artery, the dissection proceeds along the splenic artery. About 40% to 97% of patients have a posterior gastric artery supplying the posterior portion of the gastric fundus that arises from the middle portion of the splenic artery. Nodal tissue proximal to the origin of the posterior gastric artery (station 11p) should be dissected for all gastric cancers except early gastric cancers (discussed later). The nodal tissue distal to the posterior gastric artery (station 11d) should be dissected in cases that require a total gastrectomy. This portion of the dissection is easier after mobilization of the fundus by ligating short gastric arteries for the total gastrectomy.
In the retroperitoneum, the border of the right diaphragmatic crus is identified and peritoneum covering the crus is divided ( Fig. 6 yellow arrows), which provides access to the space between the anterior surface of the aorta and the nodal tissue along the lesser curvature of the stomach. Dissection of this plane from right to left mobilizes node stations 1 (right cardiac), 3 (lesser curvature), 7 (left gastric artery), and 9 (celiac) toward the stomach. The left side of the esophageal hiatus will eventually be completely exposed and the dissection plane should connect to the previous left gastric artery and the splenic artery dissection plane ( Fig. 7 ).