Although a rare presentation for testicular germ cell tumors, the optimal management for stage II disease generates considerable debate, which is, in large part, because of the potential curative role of single-modality treatment in many patients and overall excellent survival in those who require salvage treatment. Individualizing the treatment of each patient to ensure cure with minimal toxicity is challenging, given the rudimentary tools available for predicting disease relapse after initial therapy. Long-term toxicity and patient choice should be taken into account when selecting the management options.
In 2008, there were more than 52,000 new patients and 9000 deaths from testicular cancer worldwide. The incidence continues to increase in most populations across the world but this seems to be confined mostly to the populations of European ancestry. The incidence of seminoma is slightly higher than that of nonseminoma, and overall about 15% to 20% of patients present with evidence of infradiaphragmatic retroperitoneal lymphadenopathy.
Evaluation of the patient should include scrotal examination and ultrasonography to image the testis; determination of the serum tumor markers (α-fetoprotein [AFP], human chorionic gonadotropin [hCG], and lactate dehydrogenase [LDH]); and computed tomography (CT) scan of the thorax, abdomen, and pelvis. The role of other imaging modalities, such as magnetic resonance imaging and positron emission tomography (PET), for routine initial assessment is yet to be established. Tumor markers should be measured before radical inguinal orchiectomy. Occasionally, testis-sparing surgery may be performed in suitable patients. Patients with abnormal preoperative serum tumor markers should have these repeated postoperatively to aid in stage categorization. An elevated serum AFP level is considered to be associated with nonseminoma regardless of the primary pathology unless an alternative non–germ cell explanation is likely, and such patients should be managed as having a nonseminoma.
The TNM classification system should be used for staging and to determine the appropriate management. Generally, patients with retroperitoneal lymph node involvement and normal or low-level abnormality of the tumor markers are considered to have stage II disease. Options for management after orchiectomy include radiation therapy (RT) or combination chemotherapy in patients with seminoma and combination chemotherapy in those with nonseminoma, with retroperitoneal lymph node dissection (RPLND) reserved for select patients as either primary or postchemotherapy management. Overall outcomes for men who are diagnosed with stage II disease are excellent, with survival expected to exceed 95%, and patient involvement in management decision making is important, particularly because the associated short- and long-term toxicities of treatment add to the overall burden that these patients must bear. These and other survivorship issues are beyond the scope of this article and will be discussed elsewhere, but they continue to fuel some of the controversy with respect to the optimal management of the individual patient.
Stage II seminoma
After orchiectomy, about 15% of patients have enlarged retroperitoneal lymph nodes. Of these patients, 70% have small bulk disease, with lymph nodes that are 5 cm or smaller (clinical stage IIA/B). Pathologic stage II disease is generally not seen in patients with seminoma because retroperitoneal lymphadenectomy has seldom been used as the primary management. Due to the small number of patients with stage II disease, there is a lack of randomized controlled trials, and thus, treatment decisions have been guided by reports from single institutions in which patients have been managed with a uniform policy. The 2 main treatment options for stage II seminoma are RT to the retroperitoneal (para-aortic and iliac) lymph node chain or combination cisplatin-based chemotherapy. Prophylactic mediastinal radiation (PMI) was previously practiced in some institutions, although the benefit of this approach was somewhat controversial. PMI has been abandoned with the advent of effective chemotherapy and evidence that PMI was associated with unacceptable long-term toxicity.
The bulk of retroperitoneal lymphadenopathy is the single most important prognostic factor in patients with stage II disease. Lymph node size was the only factor that predicted recurrence in 95 patients with stage II seminoma treated with RT at the Princess Margaret Hospital between 1981 and 1999. The 5-year relapse-free rate in patients with nodal disease of 5 cm or less (stage IIA/B) was 91% (7 of 79 patients), compared with 44% (9 of 16 patients) in patients with bulkier disease (stage IIC). Relapses mostly occurred outside the radiation field. In this series, 6 patients died of the disease. In 31 patients who received primary chemotherapy for stage II disease, 23 had stage IIC disease. Relapses were reported in 2 patients, 1 of whom was salvaged by second-line chemotherapy. These results are consistent with other series, in which the relapse rates ranged from 6% to 13.5%, that were reported in patients with small bulk stage II disease, thus supporting the use of primary RT in this setting. Given the increase in failure after RT for bulky retroperitoneal disease (stage IIC) and that not all patients with recurrent disease were salvaged, primary chemotherapy is recommended for this subset.
As relapses almost always occur outside the radiation field, there seems to be an increased risk of distant micrometastasis in patients with stage IIC disease; the overall tumor burden should also be considered when choosing the management options, for example, a patient with multiple involved nodes extending craniocaudally along the para-aortic chain with a maximum diameter of 3.5 cm would be classified as having stage IIB disease. The risk of micrometastasis in such a patient may be higher than that in a patient with a solitary node of 3.5 cm, and thus, chemotherapy may be preferred over RT if the overall tumor burden is greater. Other factors to consider are situations in which excess morbidity induced by radiation might be anticipated, such as the need to encompass a large volume of the kidney or liver to adequately treat a laterally positioned nodal mass. A similar problem might be caused in cases of abnormal anatomy, such as a horseshoe or pelvic kidney. In such situations, chemotherapy may again be preferred.
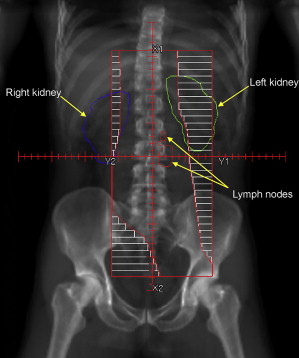
RT technique for stage II seminoma is similar to that used for stage I disease. Whereas para-aortic RT alone (excluding the pelvic nodes from the treatment volume) has been established as a treatment option for stage I disease, it has not been confirmed for stage II disease. Traditionally, a so-called dogleg-shaped RT field extending from the T10/T11 intervertebral space down to the ipsilateral obturator foramen has been used for stage II seminoma. More recently, attempts have been made to reduce this volume, recognizing that the lower internal and external iliac nodes are usually not a part of the lymphatic drainage of the testis (in the absence of prior scrotal/inguinal surgery) and thus do not need to be included in the treated volume. Thus, the radiation volume should include the gross tumor as well as the para-aortic and ipsilateral common iliac lymph nodes, which allows the inferior border of the field to usually end at the acetabulum ( Fig. 1 ). Although there is some variation, typically a dose of 25 Gy in 20 daily fractions plus a boost of 10 Gy to gross lymphadenopathy can be delivered either concurrently in 20 fractions or sequentially in 5 to 8 fractions.
The combination of carboplatin and RT has been proposed to reduce the risk of relapse associated with RT alone in patients with stage IIA/B seminoma. An updated report of this experience described 62 patients treated with 1 to 2 courses of carboplatin 4 to 6 weeks before RT. Since 1997, 29 patients have been treated with 1 course of carboplatin before RT to the para-aortic nodes alone, and no relapses have been observed. Further study of this treatment option is warranted before it can be accepted as standard practice, but it is attractive in that the rate of relapse outside the RT field may be lowered and the RT volume may be reduced. This option may allow patients to be cured while avoiding combination chemotherapy and potentially reducing the risk of late radiation–associated toxicity.
When chemotherapy is recommended as a primary treatment (or for relapse), 3 cycles of bleomycin, etoposide, and cisplatin (BEP) or 4 courses of EP (etoposide and cisplatin) are considered as standard options, which is based largely on trials of chemotherapy for good-prognosis germ cell tumors according to the International Germ Cell Consensus Classification (IGCCC) criteria. Although there was a trend toward more favorable outcomes for BEP in these trials, most patients had nonseminoma histology, and it is not clear if this can be directly applied to patients with stage II seminoma. A prospective trial of cisplatin-based chemotherapy as an alternative to RT reported on 72 men with stage IIA/B seminoma with resultant 5-year relapse-free survival of 95%. All the relapses occurred in those with stage IIB disease. Although these results are excellent, it is debatable if the incremental toxicity of combination chemotherapy is worthwhile for most patients compared with RT alone. When combination chemotherapy is chosen as the management in older men or those with poor pulmonary function, it may be preferable to omit bleomycin.
The role of carboplatin alone in IGCCC good-prognosis seminoma has been investigated in 2 randomized trials; a pooled analysis of these trials showed inferior outcomes for carboplatin and suggested that cisplatin-based combination chemotherapy remains the standard. In addition, carboplatin alone cannot be recommended as the treatment option for stage IIA/B seminoma based on a phase II trial that reported an overall failure rate of 18% at a median follow-up of 28 months.
Although many of these approaches have the aim of improving outcomes while also attempting to minimize toxicity, none have been completely successful in demonstrating an improvement. As overall disease-specific survival of patients with stage II seminoma is expected to exceed 95% regardless of the initial mode of management, only very small increases in disease control will likely be possible in the future and may well be at the expense of an increase in toxicity.
Residual Mass After RT or Chemotherapy
A residual mass seen on imaging after treatment is completed is not uncommon especially in those patients who initially present with a large nodal mass. Such masses most often contain fibrosis or necrotic material with only a few containing active tumor. Occasionally, nonseminoma elements may cause a persistent mass after therapy even in patients who have pure seminoma only in the primary tumor. RPLND in seminoma is technically challenging and associated with a higher acute morbidity after previous therapy.
Options for patients with residual masses in the posttreatment setting may include observation, surgical excision, or RT (after previous chemotherapy). PET has been advocated by some to aid in decision making but is somewhat controversial. Surgical resection of a residual mass should not rely solely on a positive scan as the presence of false positives may result in unnecessary treatment with its attendant morbidity.
As an illustration of the difficulty of surgical resection in residual masses after chemotherapy, in a published series of 55 patients, only 32 (58%) had a formal RPLND and 23 (42%) had multiple intraoperative biopsies performed, because the residual mass was deemed unresectable. Of 27 patients who had a residual mass larger than 3 cm, 8 (30%) had residual viable tumor, of whom 2 had teratoma. No patients with tumors smaller than 3 cm had viable tumor. The investigators recommended resection or biopsy of masses of 3 cm or larger. Contrary to this, other investigators suggest continued observation as long as the retroperitoneal mass continues to decrease in size after treatment.
RT has been used for residual masses after chemotherapy in men with seminoma, but the Medical Research Council Testicular Tumor Working Party published a retrospective pooled analysis evaluating the role of RT. Of 123 patients with a residual abdominal mass, 56% received consolidative RT, with no significant difference in outcome regardless of the use of RT. Given the surgical data showing a low likelihood of the presence of viable tumor in this setting, the conclusion that routine RT was not indicated is not surprising.
The use of 3 cm as a cutoff point when considering observation of a residual mass would seem to be reasonable. For those with a more bulky residual mass, immediate surgery and observation (and treatment of increasing size) are the options. The use of PET scan together with size criteria may also aid decision making in this situation.
Stage II nonseminoma
Clinical stage II nonseminoma (CS-II) refers to patients who have enlarged lymph nodes by size criteria at the time of radiographic staging, whereas pathologic stage II nonseminoma (PS-II) refers to patients with pathologic confirmation of nodal involvement after an RPLND. Although stage II only accounts for 19% of all nonseminoma diagnoses, the optimal management of this stage grouping is widely debated, which is, in large part, because of the excellent expected 5-year survival of patients diagnosed with stage II nonseminoma of more than 96%.
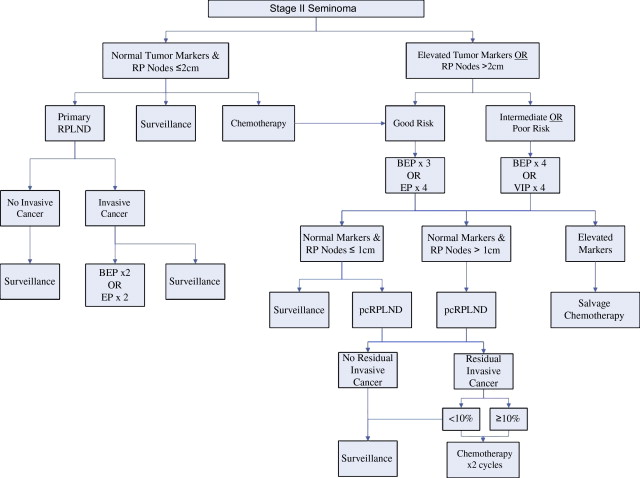
There are 3 general treatment strategies available for patients with stage II nonseminoma: primary retroperitoneal lymph node dissection (pRPLND) alone, pRPLND followed by adjuvant chemotherapy, and primary chemotherapy followed by, in the event of residual masses, retroperitoneal lymph node dissection (pcRPLND) ( Fig. 2 ). Patients with stage IIA disease (largest lymph node ≤2 cm) and negative tumor makers are a unique group, in whom close surveillance after orchiectomy may be entertained because a significant proportion of these patients have benign adenopathy. Clinical decision making for all patients with stage II nonseminoma must balance an understanding of disease biology against the expected efficacy of the available treatment strategies and their attendant long-term toxicities.
In the landmark randomized controlled trial of observation versus adjuvant chemotherapy after orchiectomy and pRPLND for patients with involved retroperitoneal lymph nodes, the rate of relapse in the observation arm was 49% compared with 2% in the adjuvant chemotherapy arm. However, there was no difference in survival between the 2 arms, because the observed patients were successfully salvaged with chemotherapy. There was a trend toward a greater risk of relapse with more extensive nodal involvement: 40% with microscopic nodal involvement, 53% with macroscopic nodal involvement of less than 2 cm, and 60% with nodal involvement of more than 2 cm. It should be noted, however, that one-third of the patients had elevated tumor markers after orchiectomy. In the modern era, patients with elevated markers would generally be treated with primary chemotherapy than with pRPLND because of the elevated risk of systemic progression associated with persistent tumor marker elevation after orchiectomy.
Patients with enlarged small-volume lymph nodes (1–2 cm) at the time of radiographic staging (CS-IIA) and normal tumor markers after orchiectomy present a unique management dilemma. The differential diagnosis for nodal enlargement includes viable germ cell cancer (PS-II), teratoma, or benign lymph node enlargement. Although there is pathologic confirmation of tumor stage, pRPLND is associated with a risk of overtreatment, because 10% to 40% of patients will be found to have uninvolved lymph nodes at the time of surgery (PS-I). As a result, close surveillance with repeat imaging and tumor marker assessment every 6 weeks until normalization of the nodes is an option for patients with CS-IIA disease and normal serum tumor markers after orchiectomy.
For patients with PS-stage IIA disease and normal pre-RPLND tumor markers, the risk of relapse is approximately 10% to 15%. Management options include surveillance with full-course chemotherapy at relapse or adjuvant chemotherapy with 2 cycles of BEP or EP. Most institutions recommend surveillance to reduce the risk of unnecessary chemotherapy for patients who are cured with pRPLND alone.
By comparison, the risk of relapse for patients with PS-IIB and PS-IIC after RPLND is substantially higher at 35% to 50% and 60% to 85%, respectively. As a result, patients with PS-IIB and PS-IIC are usually treated with 2 cycles of adjuvant chemotherapy after RPLND, which reduces the risk of relapse to less than 5%. Relapses after adjuvant chemotherapy for PS-II disease occur almost exclusively outside the retroperitoneum.
With improvements in cross-sectional imaging, patients with large involved retroperitoneal lymph nodes are now more easily identified before RPLND (CS-IIB and CS-IIC). There are no randomized clinical trials that compare pRPLND followed by adjuvant chemotherapy with primary chemotherapy for stage II disease. Comparative series suggests no difference in disease-specific survival for either approach. Recognition of risk factors associated with systemic progression after RPLND, such as lymph node size greater than 2 cm, multiple enlarged lymph nodes, and elevated AFP or hCG level, has led to an increasing use of primary chemotherapy for patients with CS-IIB and CS-IIC disease over time. Primary chemotherapy for patients with stage II disease is associated with a complete response rate of 60% to 78%. A consensus definition of complete response does not exist, but most guidelines suggest that pcRPLND is recommended for retroperitoneal masses larger than 1 cm. Necrotic debris or fibrosis is found in approximately 40% to 45% of patients with pcRPLND, immature teratoma in 40% to 45%, and viable germ cell tumor in 10% to 15%. PET after chemotherapy is not helpful in differentiating necrosis or fibrosis from other histologies. Although histologically benign, immature teratoma may grow and produce local complications. It is also associated with late viable germ cell relapses and may transform to carcinomatous or sarcomatous malignancies. When viable germ cell tumor is encountered at pcRPLND, additional chemotherapy is generally recommended to reduce the risk of systemic relapse.
An area of ongoing controversy is the management of patients with complete response after primary chemotherapy. Many authors suggest observation, because the risk of isolated retroperitoneal progression is less than 5% with long-term follow-up. In addition, pcRPLND is associated with a 6% to 8% risk of retrograde ejaculation, and the risk of pulmonary postoperative complications is increased with prior bleomycin exposure. Patients who had relapse during surveillance can subsequently be salvaged. Others recommend pcRPLND for all patients with stage II disease, arguing that radiologic response is not a reliable predictor of fibrosis or necrosis, and the incidence of teratoma identified at pcRPLND is similar for patients with residual masses larger than 1 cm versus those with residual masses of 1 cm or smaller by CT after primary chemotherapy. Regardless, cause-specific survival with either approach is excellent, and as such, patient preference should be taken into account during the decision-making process with discussion of the toxicities of surgery and radiation exposure associated with continued CT imaging.
Duration of Primary Chemotherapy
Most patients who are treated with primary chemotherapy for stage II nonseminoma are classified as good prognosis by the IGCCC criteria: AFP less than 1000 ng/mL, hCG less than 5000 IU/L, LDH less than 1.5 upper limit of normal, and no nonpulmonary visceral metastases. A landmark study of the Southeastern Cancer Study Group (SECSG) established 4 cycles of BEP as the standard for patients with disseminated germ cell tumors. The BEP regimen based on the SECSG or Indiana protocol (cisplatin 20 mg/m 2 ; etoposide 100 mg/m 2 on days 1–5; and bleomycin 30 IU on days 1, 8, and 15 for every 3 weeks) has remained the gold standard for treatment to this day. A subsequent study by the same research group demonstrated that 3 cycles of BEP were equally effective and less toxic compared with 4 cycles of BEP for patients with a favorable risk profile, defined according to their prognostic staging system that classified all patients with stage II disease as favorable risk. A joint study by the European Organisation of Research and Treatment of Cancer (EORTC) and the Medical Research Council (MRC) further established the equivalence of 3 cycles of BEP to 3 cycles of BEP followed by 1 cycle of EP for patients with good-prognosis disease defined by the IGCCC criteria. This study involved a 2 × 2 factorial design, with a second randomization of BEP to either the 5-day Indiana regimen or a 3-day regimen (cisplatin 50 mg/m 2 on days 1–2 and etoposide 165 mg/m 2 on days 1–5) for 681 of the 812 patients included in this trial. Three cycles of BEP administered by the 5- or 3-day schedule had equivalent progression-free survival, although the condensed BEP regimen was associated with greater nausea and ototoxicity.
Role of Bleomycin
Bleomycin-induced pneumonitis is a rare potentially fatal toxicity of bleomycin therapy, the risk of which increases with higher cumulative bleomycin exposure. Three cycles of BEP delivers a total bleomycin dose of 270 IU. Various trials have examined whether bleomycin can be omitted in regimens for advanced germ cell tumors. A study from the Eastern Cooperative Oncology Group randomized 178 patients with favorable-risk advanced germ cell tumors to 3 cycles of BEP versus 3 cycles of EP. The failure-free survival and overall survival were inferior in the EP arm. The EORTC randomized 419 patients with good-prognosis disease by the IGCCC criteria to 4 cycles of BEP versus 4 cycles of EP. The EP arm experienced a lower rate of complete response (87% vs 95%, P = .0075), although there were no differences in time to progression or overall survival. Of note, the dose of etoposide (360 mg/m 2 ) administered with each cycle of therapy was lower than what is now considered standard for both BEP and EP (etoposide 500 mg/m 2 ).
A large retrospective series of patients with IGCCC good-risk disease treated with 4 cycles of EP showed excellent outcomes. The Genito-Urinary Group of the French Federation of Cancer Centers randomized 270 patients with good-risk disease defined by the Institut Gustav Roussy prognostic model to 3 cycles of BEP using the Indiana regimen versus 4 cycles of EP (cumulative etoposide dose of 500 mg/m 2 ). In the French trial, both regimens achieved a similar rate of favorable response, defined as normal serum tumor markers and no residual radiographic disease after chemotherapy or pathologic complete response observed at pcRPLND. There was also no difference in 4-year event-free survival (91% vs 86%, P = .14) and 4-year overall survival (5 vs 12 deaths, P = .096) for the primary comparison. However, in the subset of IGCCC good-risk patients, there was a trend toward improved 4-year event-free survival for 3 cycles of BEP (93% vs 86%, P = .052).
Cumulative exposure to bleomycin also seems to be important. The Australian and New Zealand Germ Cell Trials Group recently reported long-term follow-up from a trial that randomized 166 patients with good-risk disease according to Memorial Sloan Kettering Cancer Center criteria to 3 cycles of B 90 E 500 P (cumulative bleomycin dose of 270 IU) versus 4 cycles of B 30 E 360 P (cumulative bleomycin dose of 120 IU). After a median follow-up of 8.5 years, the group assigned to a higher cumulative dose of bleomycin experienced improved overall survival (8-year survival 92% vs 83%, P = .037). The 4 cycles of B 30 E 360 P also received a lower cumulative dose and dose intensity of etoposide, which may confound the interpretation of the importance of bleomycin dosing in this study. Based on these findings, 3 cycles of BEP should be the preferred regimen, with 4 cycles of EP reserved for patients with contraindications to bleomycin.
Role of Carboplatin
Although less nephrotoxic and neurotoxic than cisplatin, carboplatin should not routinely be substituted for cisplatin in patients with good-risk disease. A MRC/EORTC trial randomized 598 patients with good-risk nonseminoma to 4 cycles of BEP (with 30 IU of bleomycin and 360 mg/m 2 of etoposide per cycle) or the identical regimen with carboplatin substituted for cisplatin (CEB). Patients allocated to CEB were less likely to achieve complete response (87% vs 94%, P = .009), which translated into inferior 1-year failure-free survival (77% vs 91%, P = .001) and 3-year overall survival (90% vs 97%, P = .003). A randomized trial of 4 cycles of CEB versus 3 cycles of BEP (with 120 IU of bleomycin and 500 mg/m 2 of etoposide per cycle) showed a similar rate of complete response but an increased risk of relapse with CEB. Likewise, more relapses were observed after 4 cycles of carboplatin and etoposide (CE) compared with 4 cycles of EP.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree