Fig. 22.1
Anatomy of the anorectal region: The anatomic anal canal is between the anal verge below and the dentate line above. The surgical anal canal is approximately 4 cm, with one-third below the dentate line and two-thirds above it (Illustrated by Paul Tomljanovich, MD and modified by Karen Howard, Medical Student. Courtesy of Quyen D. Chu, MD, MBA, FACS)
There are three histologic zones, the proximal glandular (rectal columnar), distal squamous (keratinizing, modified skin devoid of appendages), and squamocolumnar or transitional (nonkeratinizing) zone, which span about 0–1.2 cm above the dentate line. Squamous metaplastic tissue occurs above the dentate line and represents a transformation of the epithelium from fully developed columnar epithelium to relatively immature or incompletely developed squamous epithelium overlying columnar epithelium (6–10 cm).
According to surgeons, the anal canal extends from the rectum to the perianal skin that does not include the hair-bearing skin [4, 17]. Outside the verge is the perianal skin and encompasses a radius of 5 cm. The anal canal is 3–5 cm in men and shorter in women. It begins where the rectum enters the puborectalis sling at the apex of the anal sphincter complex and ends at the anal verge that roughly coincides with the palpable intersphincteric groove.
Pathology
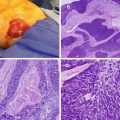
The lining of the anal canal and perianal area represents progressive transition from the digestive system to the skin, with many different types of cells and tissue. Anal cancers can arise from any of these cells and include adenocarcinoma, mucinous adenocarcinoma, undifferentiated carcinoma, small cell carcinoma, melanoma, and SCC. SCC may be keratinizing or nonkeratinizing depending on its relation to the dentate line. The majority of cancers are SCC with adenocarcinoma and melanoma making up the rest. Keratinzing SCC is rare in the anal canal above the dentate line. Variants of nonkeratinizing SCC include basaloid, large cell nonkeratinzing (transitional) and mucoepidermoid cancer. These cancers contain a mixture of squamous cells, mucin-secreting cells and cells of intermediate type [20]. Variants of SCC have similar natural history, patterns of spread, and prognosis as SCC. The other non-SCC anal cancers have distinct clinical features, natural history, and radiation sensitivity and will not be discussed.
Etiology
It was recognized in 1984 that genital and anal cancer have similar epidemiology: both are associated with the human papillomavirus (HPV) and represent sexually transmitted diseases (STD) [21]. In recent years, it became well established that the most important risk or causative factor for anal cancer is HPV [9, 22, 23]. The epithelium that is particularly susceptible to HPV infection and cancers is the transitional zone and transformational epithelium situated at variable height above dentate line [4]. Like genital cancer, HPV causes anal intraepithelial neoplasia (AIN) [squamous intraepithelial lesion (SIL)], and like cervical and vulvar intraepithelial neoplasia (CIN and VIN), AIN progresses from low-grade AIN (LG-AIN) to high-grade AIN (HG-AIN) to cancer [24]. Anal LG-AIN may not progress to HG-AIN and may regress but does not go directly to cancer without going through HG-AIN. Once established, HG-AIN rarely regresses to LG-AIN and it progresses to cancer without treatment. Although data on progression to invasive cancer is not available, it is reported in two studies with follow-up spanning nearly 20 years that about 5 % of LG-AIN undergo malignant change [25, 26]. In a recent study, malignant progression of HG-AIN occurs in 11 % of patients who were immunosuppressed for over 8 years [27].
Several risk factors are associated with anal cancer and include genetic, environmental, female gender, sexual orientation and activity, associated anogenital disease, immunosuppression (due to solid organ transplantation or HIV infection), and persistent high-risk infection with multiple HPV types (Table 22.1).
Table 22.1
Risk factors for anal cancer
Human papillomavirus (HPV 16 and 18) |
Human immunodeficiency virus infection |
Sexual behavior: |
Homosexuality |
History of anal receptive intercourse |
History of more than 10 lifetime sexual partners among heterosexual men and women |
History of anal receptive intercourse before age 30 in women |
History of genital disease: |
Cervical, vulvar, or vaginal cancer |
Sexually transmitted diseases among heterosexual men and women |
History of anogenital warts |
Immunosuppression after sold organ transplantation |
Cigarette smoking |
Inflammatory bowel disease |
Human Papillomavirus (HPV)
Infection with HPV is the first step in the pathogenesis of anal cancer. There are about 30,000 cases of anal cancer per year worldwide and 90 % are associated with HPV [28]. It is estimated that 75–80 % of sexually active Americans will likely acquire genital HPV at some point of their life, and the most common mode of transmission is sexual contact [9, 28] At times, HPV does not require direct sexual intercourse since “normal tissue trauma” and repair in the transformational zone can facilitate HPV infection. The infection causes field change throughout the perineum and may cause latent subclinical (10–40 %) or clinically apparent (1 %) disease. The virus becomes established as a result of blunted cell-mediated immune response [13, 29].
HPV subtypes 16 and 18 are strongly linked to premalignant and malignant lesions of the anus, uterus, cervix, and vulva [9, 30–33]. The prevalence of the high-risk HPV serotypes in patients with anal cancer is about 85 %, with HPV 16 and 18 occurring in the majority of the cases; HPV 16 is the most common serotype (70 %), and 80 % of affected patients have at least two HPV types [22, 34–36].
HIV
HIV represents a marker for coinfection with other STD such as HPV. HIV-positive men infected with HPV tend to carry multiple serotypes compared with HIV-negative men infected with HPV, 73 % vs. 23 %, respectively [37]. This is also true for both MSM and heterosexual men and women who do not report anoreceptive intercourse [38]. The prevalence of anal HPV infection is greater than cervical HPV infection in women who are HIV positive or have a high risk of having HIV infection [39].
SCC is the third common neoplasm observed in patients with HIV with a substantially higher incidence in MSM and in long-standing infection [40, 41]. MSM who are HIV positive have doubled the risk of developing anal cancer compared with MSM who are HIV negative [8, 14]. The risk of cancer increases with total time elapsed with CD 4+ count below 200 cells/μl and viral load >100,000 copies/ml [40, 42]. There is a 60-fold increase in relative risk of anal cancer in HIV-positive compared to HIV-negative patients [12].
With the introduction of highly active antiretroviral treatment (HAART), HIV/AIDS patients have gained improved life expectancy, but at a price of being at an increased risk for developing tumors and dying from neoplasia including AIDS-defining cancers. There is increased incidence of anal cancer in HIV-infected individuals (120-fold higher than in age- and gender-matched controls) treated with HAART as a result of increased longevity with their altered immune status [43]. SCC of the anal canal is third (8.2 %) among neoplasms in that population with higher incidence in MSM and long-standing infected people [40, 41].
Sexual Behavior
The risk of developing AIN and anal SCC is associated with sexual behavior, and the most important risk factors are lifetime number of sexual partners in homosexual and heterosexual men, engagement in anal intercourse, and presence of HIV [9]. HPV infection and high-risk sexual behavior are associated with marked increase of AIN in HIV-positive patients [44].
About 28 % of patients with anal cancer give history of genital warts as a result of HPV infection compared to 1–2 % control, and a history of receptive anal intercourse in men increases the relative risk of developing anal cancer by 33x compared with control who have colon cancer [14].
In unmarried women, history of partners with STD, 10 or more lifetime sexual partners, having at least two anal intercourse sexual partners, history of anal intercourse at young age (before the age 30), homosexuality, history of anogenital disease (warts, syphilis, gonorrhea, genital dysplasia, or cancer), and HIV infection are associated with increased risk for anal cancer [9, 29]. History of cervical or vulvar HPV infection and VIN increases the risk of anal cancer with an incidence rate ratio of 3.97–31.09 depending on age at diagnosis compared to control [32, 45].
Solid Organ Transplantation
Cell-mediated immunity is important in the host response that prohibits HPV from establishing a prolonged existence. All immunosuppression regimens used in renal transplantation are cyclosporin-based with direct suppressive effect on cellular (T cell) immunity. Among the side effects of immunosuppression is the increased incidence of neoplasms and viral infections (HPV, Epstein-Barr, and CMV) [13, 23, 46].
Smoking
Smoking is an independent factor for increasing the risk of anal cancer [14, 47, 48]. There is a 5x increased risk compared to control and the relative risks are 9.4 and 7.7 compared to nonsmoking controls [14, 47, 48]. The mechanism behind smoking and anal cancer development is unknown but may be related to interference with apoptosis and/or suppression of the immune system.
IBD
In patients with Crohn’s disease (anorectal disease), the incidence of SCC is ten times higher than in the general population [49].
Presentation
The median age of diagnosis of anal canal cancer in the United States is 60–65 years. There is a trend from older to younger patients of both genders as sexually transmitted diseases such as HIV and HPV become more prevalent. The diagnosis of SCC is often delayed because the clinical presentation is nonspecific and the coexistence of benign anorectal conditions is not uncommon. About 70–80 % of patients are initially diagnosed with benign anorectal condition, and 20 % have no symptoms [1, 5]. Invasive cancer is occasionally an unexpected finding after excision of hemorrhoids or skin tag in up to 20 % of cases [1]. AIN is present in 28–35 % of excised condylomas [50].
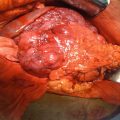
Patients may present with anorectal bleeding, pruritus, pain, and soiling. Bleeding is described in 45 % of patients, with pain and a progressive growth of an anal mass as among the presenting complaints in one of three cases (Fig. 22.2). Bleeding and presence of a mass are the complaints in 80 % of cases. Locally advanced cancers may present with incontinence (due to infiltration of the anal sphincter), perianal infection, and fistula. The average tumor size measures approximately 3–4 cm at the time of diagnosis.
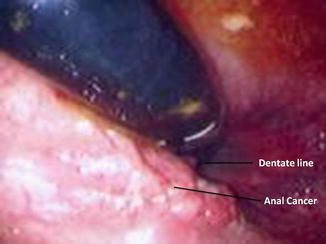

Fig. 22.2
Endoscopic view of a squamous cell carcinoma of the anal canal
Diagnostic Procedures and Staging
The widely used staging system for anal cancer is endorsed by the American Joint Committee on Cancer, 7th ed, 2010 [6] (Table 22.2). Pathologic staging was based on historical data when surgery was the gold standard and in few tumors that were locally excised. Nowadays, staging is based on clinical assessment of the primary tumor and inguinal LN, supplemented by radiologic studies such as transanal ultrasound (US) or MRI, abdominal and pelvic CT scan or MRI, PET scan, and sentinel LN biopsy (SLNBx).
Table 22.2
American Joint Committee on Cancer (AJCC) TNM staging for anal cancer (7th edition)
Primary tumor (T) | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ, Bowen disease, high-grade squamous intraepithelial lesion (HSIL), anal intraepithelial neoplasia II–III (AIN II–III) |
T1 | Tumor ≤2 cm in greatest dimension |
T2 | Tumor more than >2 cm and <5 cm in greatest dimension |
T3 | Tumor >5 cm in greatest dimension |
T4 | Tumor of any size that invades adjacent organ(s), e.g., vagina, urethra, bladder* |
*Note: Direct invasion of the rectal wall, perirectal skin, subcutaneous tissue, or the sphincter muscle(s) is not classified as T4 | |
Regional lymph nodes (N) | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph nodes metastasis |
N1 | Metastasis in perirectal lymph node(s) |
N2 | Metastasis in unilateral internal iliac and/or unilateral inguinal lymph node(s) |
N3 | Metastasis in perirectal and inguinal lymph nodes and/or bilateral internal iliac and/or bilateral inguinal lymph nodes |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis |
Anatomic stage/prognostic groups | |||
|---|---|---|---|
Group | T | N | M |
Stage 0 | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage II | T2, T3 | N0 | M0 |
Stage IIIA | T1-T3 | N1 | M0 |
T4 | N0 | M0 | |
Stage IIIB | T4 | N1 | M0 |
Any T | N2-N3 | M0 | |
Stage IV | Any T | Any N | M1 |
Clinical Assessment
Location, size, and mobility of the primary lesion are carefully assessed on examination. The anal verge remains closed when the buttocks are gently spread. Unlike perianal cancers that can be visualized by gentle traction on the buttocks, cancers of the anal canal cannot be seen in their entirety with this method alone [5]; about 50 % are <3 cm, 24 % are superficial or in situ, and 71 % invade into the muscle or fat [51].
Clinical examination for inguinal LN involvement is carefully performed since their involvement with cancer is a major independent poor prognostic factor and their presence helps gauge therapy. About 10–20 % of patients present with synchronous inguinal LN metastasis [51–53]. Tumors located laterally drain to homolateral side and those in the midline drain to bilateral groin LN [54]. Most (70–90 %) tumors are located laterally in the anal canal and have clinically negative inguinal LN at initial presentation [13, 55, 56]. Synchronous groin LN metastasis increases with tumor size and extent of the primary tumor [53]. Half of the clinically positive LNs are <5 mm in size, and of all patients presenting with palpable inguinal LN, only 50 % will turn out to be metastatic. HIV-positive patients, however, commonly present with palpable enlarged LN that are histologically positive in 50 % of cases. Needle biopsy is indicated when LNs are enlarged or are >10 mm on CT or MRI [57]. Some, however, do not advocate biopsy and recommend RT boost to the affected groin if there is palpable or radiologically suspicious LN [58].
Examination under anesthesia may be necessary to relax the sphincter and allow adequate examination. Biopsy is recommended for patients who present with pruritus or anal discharge with a suspicious raised, scaly, white plaques, erythematous, pigmented, fissured, or eczematous lesions.
Transanal US and MRI
Transanal US and MRI allow accurate determination of depth of invasion by the tumor, sphincter involvement, and local LN metastasis [18, 59]. Three-dimensional US has improved accuracy in detecting perirectal LN and tumor invasion compared to two-dimensional US [60, 61]. Proper positioning of the US probe or MRI coil may be hampered by pain and stricture. Transanal US has been mostly used in follow-up of patients treated with chemoradiation therapy (CRT) [18, 62]. Its ability to differentiate residual tumor from posttreatment fibrosis is, however, limited.
CT Scan and MRI
CT scan and MRI can delineate tumor dimension, invasion to adjacent structures, and LN involvement. MRI is more sensitive as it delineates soft tissue planes more clearly. The efficacy of CT scan and MRI to detect inguinal LN is suboptimal [49, 63]. After CRT, MRI is used with clinical evaluation to assess for therapeutic response [49].
PET Scan
PET scan has become the standard of care in staging anal canal cancer in many centers and is used as an adjunct to CT scan. It evaluates the status of LN and identifies distant metastasis. The sensitivity of PET scan for nodal disease is 89 % in comparison to 62 % for CT scan and or MRI [39]. PET identifies sites of metastasis not observed in CT scan in about 20 % of cases [39, 63–65]. It is also used in posttreatment evaluation since clinical assessment is subjective and confounded by radiation-related skin toxicity and nonmalignant residual masses [66, 67]. Furthermore, PET scan is used to assess response to treatment as it correlates with the end point of progression-free survival (PFS), overall survival (OS), and cause-specific survival [63, 68].
Sentinel LN
Management of nonpalpable inguinal LN (clinically negative) at presentation is controversial. Elective LN dissection is not widely applied since metastasis is found only in 50 % of cases and 44 % of pathologically positive LN are smaller than 5 mm [51]. Inclusion of inguinal LN in the radiation field is not standard and is empirically made according to institutional practice. Some suggest prophylactic RT whereby radiotherapy is delivered to the primary tumor, pelvic LN, and the groin with the intent to reduce the risk of late nodal metastasis [73]. Others recommend the “watch and see” policy and treating the nodal basin only when LN metastasis becomes evident after the completion of treatment of the primary tumor [61]. Others adopt a more selective approach and deliver RT based on the size of the primary tumor [74]. Sentinel LN biopsy (SLNBx) is yet another tool that can be used to improve detection of metastatic disease in clinically unsuspicious nodes. It may upstage patients with small primary anal cancer who may otherwise be excluded from radiotherapy and avoid overtreating patients who have no nodal involvement [53, 54, 71, 75–79].
Markers
Lampejo et al. in a systemic review of biomarkers found that tumor suppressor genes p53 and p21 were the only markers of prognostic value in more than one study [81].
Treatment
Management of keratinizing and nonkeratinizing anal canal carcinomas is similar. In the past, treatment included single-modality therapy, either abdominoperineal resection (APR) or RT. The treatment paradigm has changed to concurrent CRT as primary treatment and APR as salvage treatment. Below is an in-depth description of the evolution of the treatment for anal SCC.
Surgery
APR was the gold standard and treatment of choice for advanced anal cancer before 1974 [26]. The treatment was associated with an over-all survival (OS) of 40–70 %, morbidity up to 72 %, and local recurrence as high as 40 % [1, 51, 82]. With pelvic nodal involvement and tumor size larger than 5 cm, prognosis was worsened by 50 % and survival reduced to below 20 % [1, 82]. The paradigm shifted to primary CRT after Nigro published the results of neoadjuvant CRT [83]. APR became a salvage procedure offered to patients with persistent and progressive disease, and those with local recurrence and CRT-related complications such as anal stenosis and incontinence [84–90].
Local excision is suitable for in situ lesions, those that are confined to the epithelial and subepithelial connective tissue, and for small (<2 cm) well-differentiated lesions occupying <50 % of the anal circumference [51, 87]. Local excision of selected lesions is associated with a 5-year OS of 87 % and cancer-specific survival of 100 %.
Colostomy is indicated for patients who are incontinent, those who cope poorly with RT-related acute toxicity, or those who are at risk of developing a rectovaginal fistula.
Sphincter Preservation Treatments
Radiation Therapy (RT) Alone
In 1984, Cummings et al. demonstrated that survival rates with RT alone were equivalent to CRT (70 %), but primary tumor control was better with the combined therapy (60 % vs. 93 %) [91]. In a later study, they demonstrated that the 4-year actuarial cause-specific rates were better with CRT compared to RT alone (80 % vs. 68 %) [92]. Radiotherapy alone is highly effective for selected patients with small tumors (T1, T2, N0, M0) but not for those with larger tumors or those with positive LNs [93, 94]. It is also an acceptable modality for the frail and elderlies [95].
Concurrent Chemoradiation Therapy (CRT)
The Nigro protocol originally used CT as a precursor (neoadjuvant) to APR to improve survival [83]. Low-dose RT (3,000 cGy full pelvis radiation) and continuous 5-fluorouracil (5-FU infusion) and a single bolus of mitomycin C (MMC) were given during RT. Overall, 86 % of patients had at least a clinical complete response (CR), 79 % were alive and disease-free at time of analysis, and the majority had no residual tumor after APR [83]. The treatment was later modified to decrease toxicity [96]. The presence of CR subsequently led most centers to adopt CRT as primary curative treatment to avoid the morbidity and mortality associated with an APR.
Concurrent CRT evolved from a precursor to an alternative to surgery as an organ preserving, sphincter-saving, and colostomy-sparing treatment that is associated with a good outcome. It became the standard of care supported by retrospective and prospective studies, although there is no head-to-head prospective randomized trial comparing CRT to surgery. Subsequent studies focused on the selection of the chemotherapeutic agent and RT to improve the efficacy of and reduce toxicity of the treatment.
5-FU + MMC
Randomized trials conducted by the United Kingdom Coordinating Committee on cancer Research (UKCCCR) [55] and the European Organisation for Research and Treatment of cancer (EORTC) [52] demonstrated that the addition of CT yielded higher local control, colostomy-free survival (CFS), and complete remission rates than RT alone.
In the British trial (ACT I), 585 patients with T1–T4 epidermoid SCC of the anal canal or margin were randomized to RT alone (n = 290) or RT with concurrent 5-FU and MMC (n = 295) [55]. There was no significant difference in OS between the two therapies at 36 months, but CRT resulted in less local failure and decreased cancer-related risk of death (Table 22.3). With CRT, CR rate was obtained in 70 % of patients (range, 64–86 %). The long-term follow-up data (median 13 years) was recently reported and demonstrated that the full benefit with CRT can be seen at about 5 years after start of treatment and sustained at least 7 years after [97]. There was a 9.1 % increase in non-anal cancer death in the first 5 years of CRT that disappeared by 10 years. The benefits of CRT outweighed an early excess risk of non-anal cancer death.
Table 22.3
Randomized clinical trials of chemoradiation therapy for anal SCC
Clinical trial | N | Treatment | Results |
|---|---|---|---|
UKCCCR (ACT I) [55] | 577/585 | RT vs. 5-FU/MMC/RT (RT = 45 Gy; boost RT to responders) | At 3 years: |
Survival = 58 % vs. 65 % | |||
Local failure = 61 % vs. 39 % | |||
Cancer mortality = 28 % vs. 39 % | |||
Complete response rate = 70 % | |||
Early toxicity = 48 % vs. 39 % | |||
Late toxicity = no difference | |||
EORTC [52] | 110 | RT vs. 5-FU/MMC/RT | Overall survival = 58 % vs. 54 % |
Locoregional control rate = 68 % vs. 50 % | |||
Complete response rate = 54 % vs. 80 % | |||
RTOG and ECOG [58] | 310 | 5-FU/RT vs. 5-FU/MMC/RT (RT = 45–54.4 Gy) | At 4 years: |
Colostomy rate = 23 vs. 9 % | |||
Colostomy-free survival = 59 % vs. 71 % | |||
Tumor regression rate = 87 % vs. 92 % | |||
Disease-free survival = 68 % vs. 75 % | |||
High-grade toxicity = 7 % vs. 23 % | |||
644/682 | 5-FU/MMC/RT vs. 5-FU/cisplatin/RT + induction 5-FU/cisplatin (RT = 55–59 Gy) | At 5 years: | |
Overall survival = 73 % vs. 70 % | |||
Disease-free survival = 60 % vs. 54 % | |||
Local failure rate = 25 % vs. 33 % | |||
Colostomy rate = 10 % vs. 19 % | |||
Locoregional failure = 25 % vs. 33 % | |||
Distant metastasis = 15 % vs. 19 % | |||
Time to relapse = 60 % vs. 42 % | |||
Hematologic toxicity = 60 % vs. 42 % | |||
Nonhematologic toxicity = no difference (74 %) | |||
ECOG E4292 [164] | 32 | 5-FU/cisplatin/RT | Overall response rate = 92 % |
5-year OS = 69 % | |||
5-year progression-free survival = 55 % | |||
Overall high-grade toxicity = 31 % | |||
Severe hematologic toxicity = 16 % | |||
EXTRA [108] | 31 | Xeloda/MMC/RT (RT 50.4 Gy) | Complete response rate = 77 % |
No related deaths |
In all, 84 % of recurrences were detected within the first 2 years, and most anal cancer deaths occurred in the first few years (53 % in the first 2 years). Patients who survived to 5 years were considered cured since the cause of deaths thereafter were due to other causes. Only 7 % of patients developed metastatic disease without earlier locoregional recurrence. For every 100 patients, there was an expected 25.3 fewer patients with locoregional relapse and 12.5 fewer anal cancer deaths compared with 100 patients given RT alone.
The EORTC randomized 110 patients (T3–T4 or N1–N3) to RT alone or RT + concurrent 5-FU and MMC [52]. The study demonstrated no difference in the OS (58 % vs. 54 %). However, CR and colostomy-free survival (CFS) were higher in the CRT compared to RT group (Table 22.3). The locoregional control rate improved by 18 % at 5 years, and the event-free and progression-free survival rates were higher in the CRT. Unlike the UKCCCR study, the morbidity and mortality were not significantly different.
5-FU + MMC vs. 5-FU (Is MMC Necessary?)
A phase III randomized joint trial, the Radiation Therapy Oncology Group (RTOG 87-04) and Eastern Cooperative Oncology Group (ECOG 1289), was designed to determine the importance of MMC [58]. In the study, 310 patients with stages I to IIIa were randomized to 5-FU/RT or 5-FU/MMC/RT (Nigro regimen). Patients in the MMC arm compared to the 5-FU arm had significantly higher-grade 4–5 toxicity rates (23 % vs. 7 %). However, at 4 years, patients in the 5-FU/MMC arm had lower colostomy rate, higher CFS, and improved disease-free survival (DFS) than the 5-FU arm (Table 22.3). There was also higher tumor regression response in the MMC arm (92 % vs. 87 %) but no significant difference in survival (75 % vs. 68 %). Elimination of MMC resulted in almost doubling of 5-year local recurrence (from 17 % in FU/MMC to 36 % in 5-FU alone) and 17 % decrease in 5-year DFS (from 64 % in FU-MMC to 50 % in FU alone). Although 5-FU-MMC was associated with increased hematologic toxicity, this did not compromise OS. The study confirmed the superiority of MMC + 5-FU as it improved sphincter preservation and disease control, despite greater associated toxicity.
5-FU + Cisplatin (Can Platinum-Based Therapy Replace MMC?)
MMC was associated with 60 % incidence of serious (grade 3–4) toxicity, life-threatening hematologic, lung, renal toxicities, as well as an increased incidence in hemolytic-uremic syndrome [19, 65]. Cisplatinum was introduced as a possible alternative regimen given its proven efficacy in other SCC. Initial studies demonstrated high local control rates (up to 85 %) at 2 years [98–101].
The Radiation Therapy Oncology Group and the US Gastrointestinal Intergroup Randomized Trial (RTOG 98-11) conducted a randomized trial to compare RT + 5-FU/MMC and RT + 5-FU/cisplatin and to determine whether induction 5-FU/cisplatin CT followed by concurrent CRT would be more effective than standard concurrent CRT [102]. 644 of 682 patients (T2-T4, M0) received either standard CRT (5-FU/MMC) or two cycles of induction 5-FU/cisplatin followed by RT with concurrent 5-FU/cisplatin. The mean follow-up was 2.51 years. There was no difference in nonhematologic toxicity, 3- and 5-year OS, and 5-year DFS or time to relapse between the groups (Table 22.3). However, the 5-year locoregional recurrence (LRF), distant metastasis, and cumulative colostomy-free survival (CFS) rates were significantly better with the MMC-based therapy. 5-FU/MMC/RT has better DSF and lower colostomy failure (CF) rate than with induction 5-FU/cisplatin and 5-FU/cisplatin/RT. In a multivariate analysis of patients with tumor >5 cm and or node-positive disease, significant relapses occurred locoregionally (40–64 %) compared to 20 % in T2N0 or T3N0 disease [102]. In an updated long-term follow-up, it was found that RT + 5-FU/MMC had a statistically better DFS and OS than RT + 5-FU/CCDP (5-year DFS, 67.8 % vs. 57.8 %; P = 0.008; 5-year OS, 78.3 % vs. 70.7 %; P = 0.026). Additionally, the 5-FU/MMC group trended towards statistical significance for CFS (P = 0.05), LRF (P = 0.087), and CF (P = 0.074) [103].
Other studies questioned whether cisplatin could replace MMC and whether maintenance (consolidation; adjuvant) 5-FU/cisplatin would improve on those results [104, 105]. The second UK Anal Cancer Trial II (ACT II) evaluated the role of adjuvant 5-FU/cisplatin after concurrent 5-FU/cisplatin/RT or 5-FU/MMC/RT [104]. In this study, 940 non-HIV patients with SCC T1–T4 were randomized to 5-FU/cisplatin (n = 469) with RT or 5-FU/MMC (n = 471) with RT. Both groups were further randomized to receive two courses of consolidation (adjuvant) therapy, either 5-FU/cisplatin (n = 448) after CRT or no consolidation (n = 446). At 26 weeks, there was no difference in CR rate in patients receiving adjuvant CT vs. observation alone (85 % vs. 82 %). The results of the recently completed study, phase III ACT II, which showed no differences in CR, DFS, and OS in both groups, are depicted in Table 22.3 [106]. Unlike the RTOG 98-11, the colostomy rate was lower in the cisplatin arm (11 % vs. 14 % with MMC). As in RTOG 98-11, grade 3–4 acute hematologic toxicity occurred significantly more often in the MMC arm (25 % vs. 13 %), whereas nonhematologic toxicity did not differ between the two arms (60 % vs. 65 %) [106].
The European Action Clinique Coordonnees en Cancerologie Digestive (ACCORD-03) phase III trial evaluated the benefit of cisplatin-based neoadjuvant CT prior to concurrent 5-FU/cisplatin/RT and assessed the impact of higher RT boost to responding patients [high-dose boost (25 Gy vs. standard dose boost (15 Gy)] on CFS in patients with advanced anal cancer [107]. The study showed no benefit for the addition of cisplatin-based neoadjuvant CT prior to CRT or from increase in RT boost (Table 22.4).
Table 22.4
Neoadjuvant vs. adjuvant chemotherapy
Study | N | Treatment | Outcome |
|---|---|---|---|
940 | Cisplatin group | CR: similar (94 %) | |
5-FU/cisplatin/RT | OS: similar (85 %) | ||
± adjuvant 5-FU/cisplatin | 3 year RFS: similar | ||
T1–T2 = 75 % | |||
MMC group | T3–T4 = 68 % | ||
5-FU/MMC/RT | Toxicity: | ||
± adjuvant 5-FU/cisplatin | Nonhematologic: similar 60 % vs. 65 % | ||
Hematologic: MMC > cis 13 % vs. 25 % | |||
ACCORD 03 [107] | 307 | NACT + 5-FU/cisplatin/RT | 5-year CFS: |
Standard boost RT (group A) | Group A and B = 76.5 % | ||
High-dose boost RT (group B) | Group A and C = 73.7 % | ||
No NACT + 5-FU/cisplatin/RT | |||
Standard boost RT (group C) | Group C and D = 75 % | ||
High-dose boost RT (group D) | Group B and D = 77.8 % | ||
Standard boost RT = 15 Gy | |||
High-dose boost RT = 25 Gy |
Capecitabine and MMC
In gastrointestinal cancer, oral capecitabine (Xeloda®) is at least as effective with similar toxicity profile as intravenous 5-FU. The efficacy and toxicity of capecitabine in the treatment of anal SCC was explored in a multicenter phase II study (EXTRA) that replicated ACT II treatment but substituted capecitebine for 5-FU [108]. Thirty one patients were treated with CRT using intravenous MMC on day 1 and oral capecitabine on each RT treatment day in two divided doses (825 mg/m2 BID). The combination of MMC/capecitabine was well tolerated. Complete CR occurred in 77 % of patients and partial response occurred in 16 %, and there were no treatment-related deaths (Table 22.3).
In another phase I study of 18 patients with locally advanced anal cancer who were treated with intensity-modulated RT (IMRT) and concomitant capecitabine and MMC, 83 % of patients achieved CR. At a follow-up of 28 months, none of the patients with CR relapsed [109]. The predominant acute toxicity (grade ≥3) was radiation dermatitis (50 %) [109].
Cetuximab and Cisplatin
Early results of treatment with EGFR inhibitor (Cetuximab) in metastatic anal SCC are promising [110–113]. Patients with KRAS wild-type showed partial response or at least stabilization of the tumor, whereas patients harboring KRAS mutation had progressive disease [112, 113]. A phase I study of cetuximab with cisplatin and RT for locally advanced anal cancer showed 78 % of patients (7/9) achieved CR [114].
HIV Status and CRT
HIV-negative patients with anal cancer treated with concurrent CRT often required a break from RT and dose reduction of at least one CT agent and have a local failure rate of 30 % and a 5-year OS near 70 %; more than 50 % succumb to death for which the causes are unrelated to anal cancer [52, 55, 58, 102, 115]. HIV-negative patients with anal cancer differ from HIV-positive patients by age (60–65 years vs. 40–45 years), male gender (35–40 % vs. 90–95 %), and homosexuality [116–118]. Currently in the Western world, up to 50 % of patients with anal SCC are relatively young (40–60 years) male homosexuals under highly active antiretroviral therapy (HAART) [119].
In the pre-HAART era, HIV-positive patients were excluded from major studies. Small studies, however, have shown that HIV-positive patients had poor tolerance to MMC-based CRT protocol, experienced frequent local recurrence, but had satisfactory local control and survival rates [40, 41, 117, 120–123]. Despite poor tolerance, standard CRT was given whenever possible, especially when the CD 4+ count was >200/mm3 [123]. Many of these young patients eventually died of AIDS with or without evidence of residual anal cancer since the median survival with the diagnosis of AIDS was 17 months. In one study where many patients did not receive standard CT because of fear of significant hematologic toxicity, after 38 months follow-up, 40–50 % had local recurrence, 50 % were alive and disease-free, and 50 % died from complications of AIDS [115]. Acute toxicity was frequent (>50 %). In another study, the median time to cancer-related death was 1.4 years (vs. 5.3 years in HIV-negative patient) [117].
In the HAART era, HIV-positive patients were able to tolerate CRT if CD 4+ count >200 [123]. In a multicenter cohort study and retrospective analysis of 40 HIV-positive patients (on HAART) and 81 HIV-negative patients treated with either CRT or RT alone, the CR rate was high in both groups (92 % vs. 96 %), although HIV-positive patients were more likely to experience grade 3–4 skin and hematologic toxicity [120]. The 5-year OS was similar in both groups (61 % vs. 65 %), but the locoregional recurrence was higher in the HIV-positive patients (61 % vs. 13 %), and the majority of HIV-positive patients died of anal cancer [120]. In the Veterans Affairs study, the authors noted that survival was equivalent between HIV-positive and HIV-negative patients (overall 4-year survival 66 % vs. 62 %) [118]. Overall, HIV-positive individuals with anal SCC in the HAART era carry a 50 % risk of local recurrence and 33 % risk of dying from the cancer [40].
Radiotherapy: Standard RT vs. Intensity-Modulated RT (IMRT)
Standard Radiotherapy [Conventional 3D-Conformal Radiotherapy (3D CRT)]
The RT that has been used is 3D conformal radiation therapy (3D CRT). It delivered RT using opposed anteroposterior and posteroanterior fields during a 6-week period with a therapeutic break between sequences [94, 124–126]. The delivery covered gross disease and elective pelvic and inguinal LN including extensive portions of the bowel, bladder, and perineum. The total dose of RT continues to be evaluated. Nigro initially utilized low-dose RT, 30 Gy [83]. However, escalating or higher doses has been shown to improve locoregional recurrence [127, 128]. Dose 50–55 Gy is effective in obtaining local control and eradication of the tumor, resulting in a cure in 70–90 % of patients [1, 124, 127–129]. Local recurrence dropped to less than 10 % and 5-year survival improved to 60–90 %. Escalating the dose up to 60 Gy is associated with 90 % locoregional control at 5 years and improves survival in patients with T3 and T4 tumors [129, 130]. The benefit of a dose >60 Gy is doubtful and is associated with complications [88, 89, 131–133].
Extensive portions of the bowel, urinary bladder, and perineum and large volume of bone marrow are included within each RT field arrangement, leading to increase in acute toxicity and chronic sequelae that can pose life-threatening complications and adversely affect quality of life [53, 88, 134–136]. Death from RT toxicity is reported in 2–2.7 % of cases [53]. Hematologic and nonhematologic toxicities are observed in 42–74 % of the patients, and the incidence of grade ≥3 GI and skin toxicity ranged from 34 to 48 % [52, 55, 58, 102–104, 106, 137]. Irradiation to groin LN cancer results in significant early and late toxicity: external genitalia edema, epidermolysis with super infection of the skin, inguinal fibrosis, osteonecrosis of femoral head, femoral head fracture, small bowel injury, lymphedema of lower extremities, edema of genitalia, and stenosis of iliac artery that occur in 33 % of irradiated patients [89, 134–136]. There is a threefold increase in the risk of pelvic fractures in elderly women [138]. High-dose radiation is associated with anal stenosis, ulcers, and necrosis that may require a colostomy in up to 6–12 % of patients [88, 89, 124]. With increasing doses of RT, the need for a diverting colostomy increases, and with a dose >65 Gy, necrosis of the anal canal occurs [132]. Potential toxicity of 3D CRT results in significant dose reduction of CT and increases incidence of treatment breaks (gap) and overall treatment time. Prolonged treatment with frequent treatment interruptions is associated with a poor prognosis and local control rate [94, 133, 139–141]. To minimize the risk of RT toxicity, the radiation field may be reduced, or multifield techniques are used to deliver the dose of RT more conformally to spare normal tissues.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree