Spindle Cell Lesions
A variety of reactive and neoplastic lesions of the breast are characterized by a proliferation of spindle cells (Table 11.1).1, 2, 3, 4 and 5 When any spindle cell lesion is encountered in the breast, the diagnosis of spindle cell carcinoma (a type of metaplastic carcinoma) should always be considered and that diagnosis should not be dismissed unless there is histologic and/or immunophenotypic evidence to exclude it. The possibility of a phyllodes tumor should also be given consideration because the epithelial component may be difficult to identify in some cases, particularly in malignant lesions characterized by prominent stromal overgrowth or in small biopsy samples (see Chapter 6). In fact, the definitive categorization of any spindle cell lesion of the breast may be difficult or impossible due to the limited sampling afforded by core-needle biopsy; therefore, a cautious approach regarding the diagnosis of spindle cell lesions based on core-needle biopsy specimens is prudent.
Many spindle cell lesions that occur in other anatomic sites may be seen in the breast. This chapter will focus on those lesions that are most likely to be encountered in clinical practice or that produce particular diagnostic difficulties.
SPINDLE CELL CARCINOMA
As noted in Chapter 10, some carcinomas of the breast included within the broader category of metaplastic carcinoma are composed of spindle cells. These tumors may occur in pure spindle cell form or may consist primarily of spindle cells with associated glandular, squamous, or heterologous elements (most often chondroid or osseous). Tumors of this type have been variously designated metaplastic carcinoma, sarcomatoid carcinoma, and spindle cell carcinoma.1, 2 and 3,5, 6, 7, 8, 9 and 10 Spindle cell carcinomas are uncommon and account for <1% of invasive breast cancers.
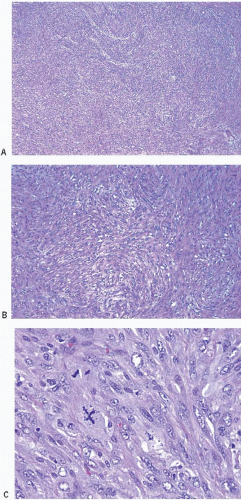
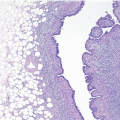
Upon gross examination, the tumors are most often gray or white, firm masses that are frequently circumscribed. Microscopically, the appearance of the spindle cells can vary from bland to highly pleomorphic and may show fascicular, fasciitis-like, storiform, or haphazard growth patterns
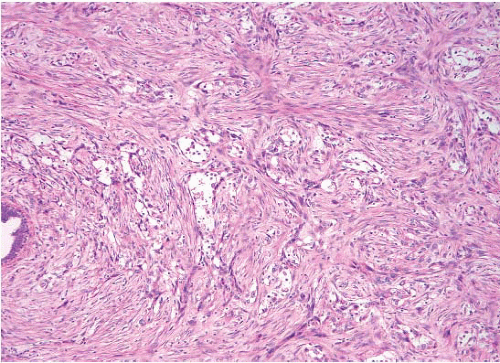
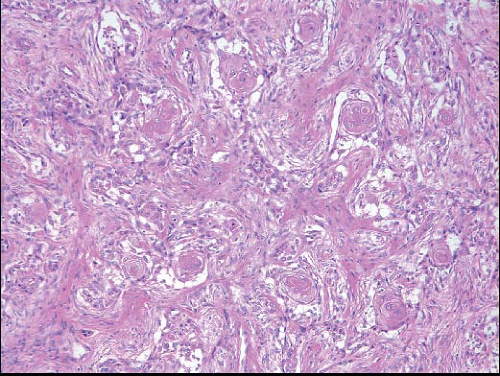
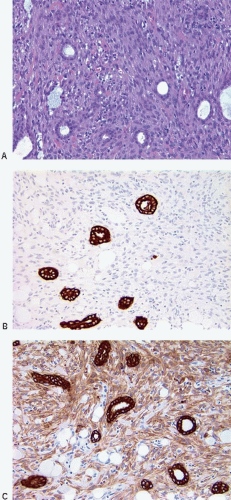
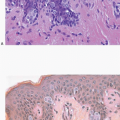
(Fig. 11.1, e-Figs. 11.1, 11.2 and 11.3). The mitotic rate is highly variable. The borders of the lesion are usually infiltrative, with entrapment of mammary ducts and lobules and irregular infiltration of adjacent adipose tissue (Fig. 11.2), but some cases exhibit pushing margins. Higher grade lesions tend to obliterate the normal breast architecture. In some cases, the spindle cells aggregate into small clusters that exhibit a more epithelioid appearance or merge with an overt epithelial component. Areas suggestive of vascular spaces may sometimes be seen (Fig. 11.3, e-Fig. 11.4) and areas of squamous differentiation are not infrequent (Fig. 11.4, e-Fig. 11.5). Small foci with heterologous chondroid or osseous differentiation may be observed.
(Fig. 11.1, e-Figs. 11.1, 11.2 and 11.3). The mitotic rate is highly variable. The borders of the lesion are usually infiltrative, with entrapment of mammary ducts and lobules and irregular infiltration of adjacent adipose tissue (Fig. 11.2), but some cases exhibit pushing margins. Higher grade lesions tend to obliterate the normal breast architecture. In some cases, the spindle cells aggregate into small clusters that exhibit a more epithelioid appearance or merge with an overt epithelial component. Areas suggestive of vascular spaces may sometimes be seen (Fig. 11.3, e-Fig. 11.4) and areas of squamous differentiation are not infrequent (Fig. 11.4, e-Fig. 11.5). Small foci with heterologous chondroid or osseous differentiation may be observed.
TABLE 11.1 Major Differential Diagnostic Considerations of Spindle Cell Lesions of the Breast, Based on Cytologic Appearance of Spindle Cells | ||||
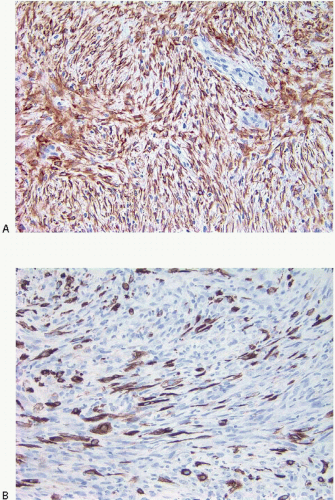
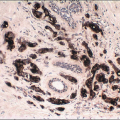
|---|---|---|---|---|
|
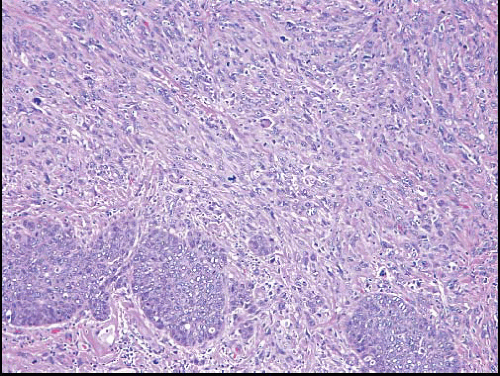
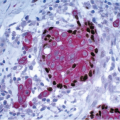
Foci of conventional types of invasive breast carcinoma and/or ductal carcinoma in situ (DCIS) may be seen in association with the spindle cell component and, when present, provide useful clues to the epithelial nature of the tumor (Fig. 11.5, e-Fig. 11.6). However, in pure spindle cell carcinomas without morphologic evidence of an epithelial component or associated DCIS, immunohistochemical stains for cytokeratin and other markers may be required to arrive at the correct diagnosis.4,10,11 It should be noted, however, that cytokeratin immunoreactivity may be quite focal and a panel of anticytokeratin antibodies may be required to demonstrate cytokeratin positivity. In our experience, broad-spectrum cytokeratin antibodies (such as antibody MNF116) and antibodies to high-molecular-weight/basal cytokeratins (such as antibody 34βE12 or antibodies to cytokeratin 5/6) are the most sensitive for the detection of cytokeratin expression in this setting (Figs. 11.6 and 11.7, e-Fig. 11.7).4,9 The neoplastic cells also commonly express p63 as well as vimentin, smooth muscle actin, and muscle-specific actin (Fig. 11.8, e-Fig. 11.8). Expression by these tumors of basal cytokeratins, p63, actins, and other markers commonly associated with myoepithelial cells12, 13 and 14 supports a basal epithelial and/or myoepithelial phenotype for these tumors and blurs the distinction between spindle cell carcinomas and lesions that have been previously categorized as myoepithelial carcinomas or malignant myoepitheliomas. In fact, this distinction now appears to be one of semantics.15 Recent gene expression profiling studies have demonstrated that some of these tumors have a basal-like phenotype and cluster in the “basal-like” group, whereas others show features of epithelial-mesenchymal transition and cluster in the “claudin low” group.16 Spindle cell carcinomas are typically negative for estrogen receptor and progesterone receptor expression and for HER2 protein overexpression (triple negative).
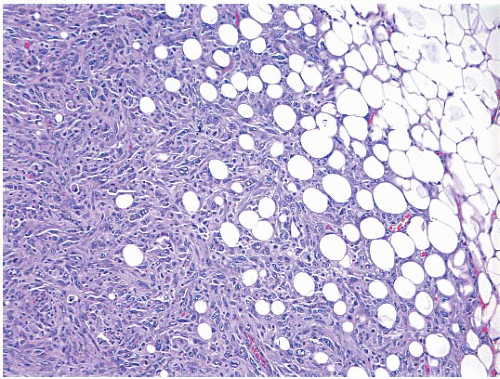 FIGURE 11.2 Spindle cell carcinoma. At the border of the lesion, there is irregular infiltration of adjacent adipose tissue producing a “lace-like” pattern. |
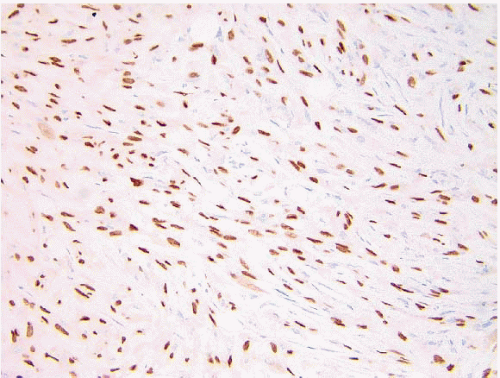 FIGURE 11.8 Spindle cell carcinoma. This p63 immunostain demonstrates reactivity in most of the tumor cell nuclei. |
One type of spindle cell carcinoma worthy of particular note is the low-grade fibromatosis-like metaplastic carcinoma.17,18 These lesions, which may arise either de novo or in association with papillomas and benign sclerosing lesions,19 are composed of bland spindle cells resembling those seen in scars or fibromatosis (Fig. 11.9). In contrast to the cells of scars or fibromatosis, however, the spindle cells comprising these lesions show expression of cytokeratin, and some cases show epithelioid cell clusters or small foci of squamous differentiation. These tumors have been reported to be associated with a high rate of local recurrence. Although no instances of metastasis were observed in the initial report of these cases,17




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree