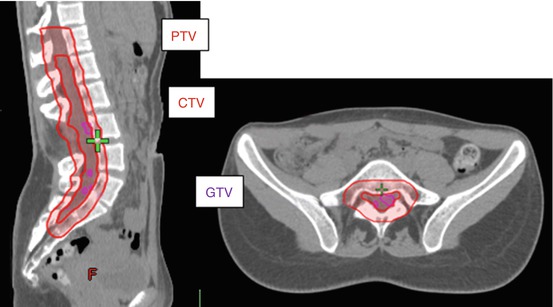
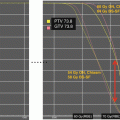
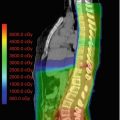
Fig. 18.1
Fifteen-year-old girl with known diagnosis of neurofibromatosis type I was incidentally found to have lesions in distal thecal sac during surveillance imaging (sagittal T1 sequence post-gadolinium administration demonstrated several enhancing masses (arrows)). Pathology after resection was consistent with grade II ependymoma
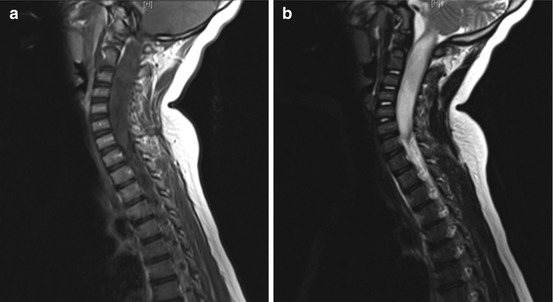
Fig. 18.2
Expansile cervical cord lesion in a 7-year-old child, nonenhancing on T1 post-contrast sagittal image (a) and hyperintense on T2 sagittal sequence (b), pathology from biopsy was consistent with anaplastic astrocytoma
In evaluating spinal cord tumors, it is important to image the entire length of the spinal canal. It is important not to miss rare, but clinically important “skip” lesions sometimes seen in astrocytomas and ependymomas, and/or subarachnoid dissemination, more commonly found in malignant gliomas and ependymomas (Hardison et al. 1987; Cohen et al. 1989). Brain imaging should be performed in multifocal and disseminated cases, as there is a risk of rostral disease spread. The presence of multiple discrete tumors, along with other stigmata, is commonly associated with neurofibromatosis.
18.5 Workup
Medical history should focus on details of presenting symptoms including duration. Long-standing symptoms are more indicative of low-grade tumors while rapidly evolving ones raise concern for a more aggressive neoplasm. Additional history, including pre-existing genetic conditions and history of ionizing radiation exposure, cannot be overlooked. Inheritable genetic syndromes should be recognized in the family history. Attention during the physical examination should be directed to both upper and lower motor neuron signs, as well as sensory function. The constellation of neurological signs, such as Brown-Séquard syndrome, can help to localize the lesion. Imaging evaluation gives the best delineation of tumor process, and should include the entire neuraxis. As discussed above, MRI has become a gold standard in imaging of spinal tumors and should be considered first, when available. Cerebrospinal fluid (CSF) examination for protein levels, cytology, and infectious cultures, among other factors, can help diagnostic workup, particularly when nonneoplastic processes are still considered in diagnosis. Cytological detection of tumor cells can confirm the presence of disseminated tumor and can be complementary to spinal imaging. Ultimately, biopsy or resection, when feasible, can establish the most accurate diagnosis.
18.6 Acute Management
Spinal cord tumors commonly grow contiguously across the spinal cord and can result in significant mass effect on neurons and lead to a functional loss (Peschel et al. 1983; Reimer and Onofrio 1985; Hardison et al. 1987). Steroids are commonly prescribed to patients exhibiting symptoms from tumor mass effect, but laminectomy with or without tumor decompression is a very important intervention. Steroids should be administered carefully, as it could alter diagnosis in certain histologies such as lymphoma or other infectious agents.
18.7 Treatment
The benefit from postoperative radiotherapy in low-grade intramedullary spinal cord tumors is debatable. Data is limited to institutional case series, and there is no randomized study that has evaluated the use of postoperative radiotherapy (Nadkarni and Rekate 1999). While the extent of resection has more consistently been shown to be a significant prognostic factor in tumor control probability for low-grade lesions, tumor histology is the most important predictor of a patient’s outcome (Whitaker et al. 1991, Innocenzi et al. 1996, Goh et al. 1997, Ahmed et al. 2014). A handful of studies demonstrated a trend toward improved tumor local control with the use of adjuvant radiotherapy when compared with surgical resection alone (Nadkarni and Rekate 1999, Guss et al. 2013). With regard to grade II ependymomas in children, radiotherapy is most commonly given after subtotal versus gross-total resection; this practice trend in North America has not significantly changed over the past three decades (Lin et al. 2014). There is also limited, but convincing evidence on radiation treatment achieving durable local control in patients without any surgical resection (O’Sullivan et al. 1994, Guss et al. 2013).
For high-grade spinal cord tumors, outcome overall remains dismal. Therapeutic advances in the management of high-grade gliomas of the spinal cord have been hampered by the rarity of incidence and the lack of uniform treatment. Despite the lack of evidence for improved outcome, trimodality approach including maximum safe resection, adjuvant radiotherapy and chemotherapy is commonly used (Merchant et al. 1999).
18.7.1 Role of Surgery
Surgical management plays a very important role in spinal cord tumors for establishing diagnosis while achieving maximum safe resection for improved local control. Laminectomy or laminoplasty, as appropriate, is typically performed to access the lesion. Extramedullary intradural tumors are more readily accessible and can be completely removed for diagnostic and treatment purposes (Jenkinson et al. 2006). When it comes to intramedullary lesions, intraoperative tumor resection is based on whether a plane of dissection can be identified, which is often dependent on tumor histology. For instance, ependymomas commonly demonstrate a well-demarcated margin between tumor and spinal cord tissues, while astrocytomas can have infiltrative borders; however, for each histological type, there is considerable variability in the presence or absence of identifiable tumor planes (Garces-Ambrossi et al. 2009). The presence of identifiable tumor planes carries positive prognostic significance regardless of tumor type, suggesting that this may offer valuable prognostic information regarding biological aggressiveness and subsequent recurrence. Low-grade spinal cord astrocytomas, in spite of their infiltrative behavior, are amenable to radical surgical resection. Gross-total resection (GTR) has been associated with improved prognosis for low-grade intramedullary tumors such as ependymoma and hemangioblastoma (McCormick et al. 1990, Hanbali et al. 2002, Stephen et al. 2012, Karikari et al. 2015).
Gross tumor resection can significantly affect tumor control and survival for most types of spinal cord tumors, and should be attempted when deemed safe (Garces-Ambrossi et al. 2009). Typically, gross-total and near-total resection is reported in 6–46% of cases with a trend toward increased radical resection in more recent times (Reimer and Onofrio 1985, Allen et al. 1998, Garces-Ambrossi et al. 2009). Subtotal resection (STR) can be achieved in up to 50% of cases (Kutluk et al. 2015). Surgical complication rates are low in the hands of skilled neurosurgeons; however, preoperative functional status is a predictor of good postoperative function (Jenkinson et al. 2006). It has also been shown that significant intraoperative changes of somatosensory evoked potentials can predict postoperative deficit.
Modern surgical techniques with microscopy, intraoperative ultrasonography, and electrophysiological monitoring (sensory and/or motor evoked potentials) can greatly improve the probability to identify tumor margin and achieve total resection with less functional damage (Zentner 1991, Constantini et al. 1996, 2000, Hoshimaru et al. 1999, Houten and Weiner 2000). Intraoperative ultrasound can be used as a tool to demarcate tumor for resection. The use of electrophysiological monitoring has become common practice and can provide the surgeon with continuous intraoperative information about the integrity of the spinal tracts in order to facilitate an aggressive resection with minimal morbidity and to predict postoperative function. Somatosensory evoked potentials (SSEPs) monitor the function of the posterior columns, and its intraoperative monitoring can affect postoperative outcome (Kearse et al. 1993). Motor evoked potentials (MEPs) are a more recently developed technique using scalp and epidural electrodes to provide “real time” information about the integrity of the motor tracts.
18.7.2 Role of Chemotherapy
Experience using chemotherapy is limited. The rationale for using it is mainly based on extrapolation from similar tumors in the brain.
The standard treatment of spinal cord astrocytomas is surgery, followed by radiotherapy for incompletely resected or high-grade tumors. Owing to the major consequences of radiotherapy on the spine in childhood, alternative therapies have been explored. The potential role of chemotherapy in the management of spinal cord astrocytoma remains to be defined. A small case series demonstrated benefit in both low- and high-grade tumors (Lowis et al. 1998). The Children’s Cancer Group (CCG) 945 High-Grade Astrocytoma Committee devised a pilot study to collect natural history information and explore the benefit of an experimental multimodality treatment in children with newly diagnosed high-grade astrocytomas arising within the spinal cord (Allen et al. 1998). Patients were assigned in a nonrandom fashion to the experimental regimen of an 8-drugs-in-1 regimen consisting of vincristine 1.5 mg/m2, lomustine 100 mg/m2, procarbazine 75 mg/m2, hydroxyurea 3000 mg/m2, cisplatin 90 mg/m2, mannitol 12 g/m2, cytarabine 300 mg/m2, dacarbazine 150 mg/m2, and methylprednisolone 300 mg/m2 for three doses (Allen et al. 1998). A centralized neuropathology review was used to confirm the diagnosis of high-grade astrocytoma in 13 of the 18 children: anaplastic astrocytoma (eight patients), glioblastoma multiforme (four patients), and mixed malignant glioma (one patient). Diagnoses were discordant in five patients. There were eight boys and five girls in the group with confirmed diagnoses, with a median age of 7 years (range 1–15 years). The extent of resection was confirmed by computerized tomography or magnetic resonance (MR) evaluation in five of 13 patients. There were six gross-total or near-total resections (>90%), four partial or subtotal resections (10–90%), and three biopsies. Six patients showed evidence of leptomeningeal metastases at diagnosis based on staging MR examinations. Eight of the 13 patients completed at least eight of the prescribed 10 cycles of chemotherapy; five received craniospinal radiotherapy and five had spinal radiotherapy. The 5-year progression-free and overall survival rates for the 13 children were 46 ± 14% and 54 ± 14%, respectively.
Eight children with unresectable or recurrent intramedullary glioma were treated on a French Society of Paediatric Oncology (BB SFOP) protocol of a 16-month chemotherapy regimen with carboplatin, procarbazine, vincristine, cyclophosphamide, etoposide, and cisplatin. Six children had progressive disease following incomplete surgery and two had a postoperative relapse. Three patients had leptomeningeal dissemination at the outset of chemotherapy. Seven of the eight children responded clinically and radiologically while one remained stable. At the end of the BB SFOP protocol, four children were in radiological complete remission. After a median follow-up of 3 years from the beginning of chemotherapy, all children but one (who died from another cause) are alive. Five patients remain progression-free, without radiotherapy, 59, 55, 40, 35, and 16 months after the beginning of chemotherapy. The efficacy of this chemotherapy in patients with intramedullary glial tumors calls for further trials in this setting, especially in young children and patients with metastases. While chemotherapy may delay the need to radiotherapy administration in low-grade glial tumors, most of the patients would need radiotherapy to achieve tumor control (Gajjar et al. 1995, Doireau et al. 1999, Merchant et al. 2000, Bian et al. 2013, Schniederjan et al. 2013). The role of chemotherapy in ependymoma, studied exclusively for intracranial tumors, has proven controversial due to mixed results (Evans et al. 1996, Timmermann et al. 2000, Garvin et al. 2012, Venkatramani et al. 2013, Strother et al. 2014); however, there is renewed interest in investigating its role for WHO grade II and III tumors.
18.7.3 Role of Radiation Therapy
The use of postoperative irradiation for spinal cord tumors has been controversial, and largely inconsistent. There may be an increased trend toward using upfront chemotherapy for low-grade spinal astrocytomas. Radiotherapy is commonly used for progression or recurrence. In some series, radiotherapy was shown to improved progression-free survival while others report no advantage (Bouffet et al. 1998, Ahmed et al. 2014).
For low-grade tumors, surgery and irradiation after incompletely resected spinal astrocytomas can result in survival rates of 50–60% at 5 years (Houten and Weiner 2000, Jenkinson et al. 2006, Ahmed et al. 2014). Although lacking conclusive data, a rational approach includes maximal surgical resection. For incompletely resected tumors, one can support observation for low-grade gliomas (pilocytic histology), especially in prepubertal children where the risk-to-benefit ratio may favor delaying radiation intervention. If one elects to observe a child with suspected or definite residual disease, it is important to commit to a later second surgery when feasible, as well as irradiation, unless the second surgery results in imaging-confirmed total resection, at the time of disease progression.
For spinal cord ependymomas, there is evidence supporting surgery alone for intramedullary tumors and for initial management of cauda equina tumors (McCormick et al. 1990). The indolent nature of myxopapillary tumors may argue for favoring observation after good resection in a young child; however, there is evidence that children with myxopapillary ependymoma experience a shorter time to recurrence and higher rates of dissemination with surgery alone (Bagley et al. 2009, Feldman et al. 2013, Bandopadhayay et al. 2016). Adjuvant radiotherapy can significantly improve local control and progression-free survival and should be considered strongly, especially after STR (Schild et al. 1998, Akyurek et al. 2006, Agbahiwe et al. 2013). Although the impact of histologic grade in ependymomas is apparent in adult studies, higher grade ependymomas of the spinal cord are uncommon in children. Extrapolation from the adult data suggests a role for postoperative irradiation for such lesions. It is evident that patients with grade II ependymoma, especially after subtotal resection, may benefit from postoperative radiotherapy (Lin et al. 2014).
18.8 Target Delineation
18.8.1 Radiotherapy Volume
Radiation therapy target volume definitions have evolved significantly in the last two decades based on improved imaging technology. Full neuraxis imaging is warranted, and MRI gives the best anatomical delineation of tumor extent. Tumor extent in the craniocaudal direction can be delineated based on MRI images; image registration should be employed when available to allow accurate delineation of target volumes. Localized intramedullary astrocytomas are generally treated with focal fields targeting the gross tumor and/or tumor bed, based on preoperative and postoperative MRI. Local fields are also indicated for spinal ependymomas (Akyurek et al. 2006, Agbahiwe et al. 2013). Recent studies suggest regional irradiation with a slightly more generous margin and including entire distal thecal sac. In the postoperative setting, target volume definition should start with an analysis of preoperative image sets to appreciate the full extent of disease in the craniocaudal direction; additionally, postsurgical changes should be incorporated in clinical target volume since they may extend beyond original tumor levels and commonly reflect an actual tumor plane. The additional margin for covering microscopic disease extent is created (clinical target volume or CTV); in the longitudinal direction, it is typically 2–3 cm and radially typically includes the entire spinal canal to the body edges of the vertebral body. For astrocytomas, one typically sees decompression or obliteration of the rostral and caudal cystic components after resection of the solid tumor. In such instances, it appears that radiation therapy can be limited to the solid tumor bed. Planning target volume expansion will depend on patient setup, immobilization, and onboard imaging capabilities of treating institution. Typically, the PTV ends up being 0.5–1 cm from the CTV, respecting symmetrical coverage of vertebral bodies in skeletally immature child (Fig. 18.3).