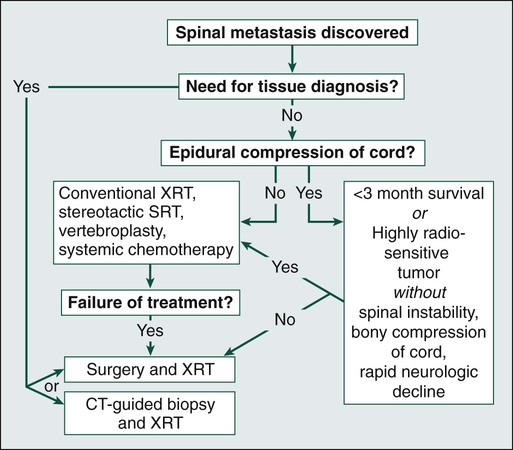
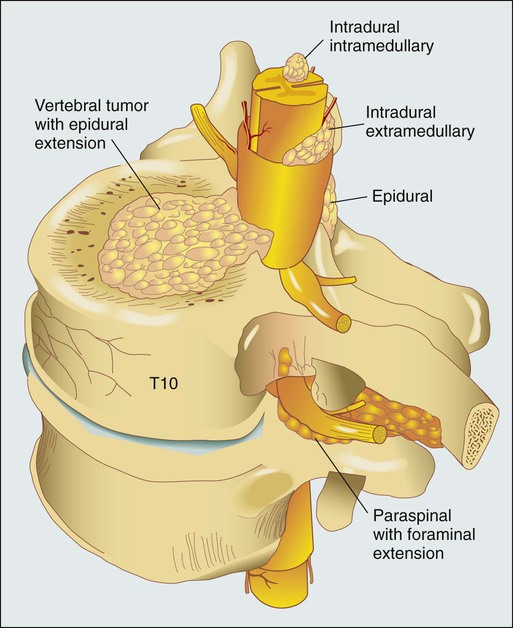
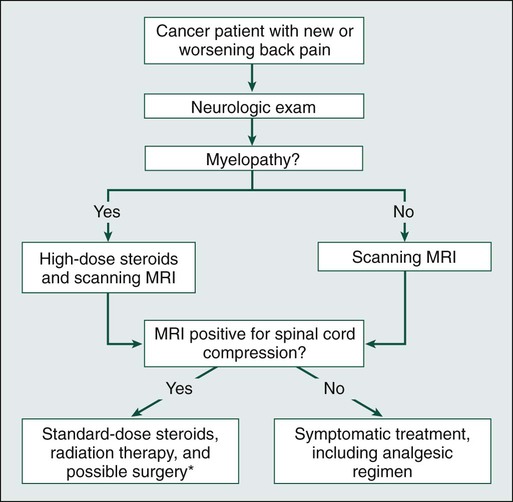
Daniel M. Sciubba, Ali A. Baaj and Ziya L. Gokaslan • Spinal cord compression is diagnosed in more than 30% of all patients with disseminated cancer, and roughly 5% to 10% of all patients experience cord dysfunction. • Back pain is the most common presenting complaint and almost always precedes neurologic dysfunction. • Spinal cord compression has a devastating effect on quality of life and is frequently diagnosed late. • Compression results from an epidurally located tumor or from bony fragments generated by pathologic fractures. • Constant compression leads to spinal cord arterial compromise, venous occlusion, vasogenic edema, and demyelination with subsequent clinical deterioration. • Any new or worsening back pain in a patient with cancer mandates urgent radiographic evaluation. • Any evidence of myelopathy may signal impending catastrophic loss of cord function. • Magnetic resonance imaging of the entire spine is the most rapid and cost-effective means of diagnosis and should be the first procedure performed. • Corticosteroids, conventional radiation therapy, and decompressive surgery are the mainstays of treatment. • Although treatment of metastatic spinal lesions is considered palliative, aggressive medical and surgical treatment can significantly improve pain, neurologic function, and quality of life. Spinal metastases have long been a significant source of morbidity in patients with systemic cancer. Regional pain, pathological compression fractures, deformity, and spinal cord compression with ensuing neurologic compromise are problems that commonly must be managed in both patients with advanced cancer and patients with isolated metastatic vertebral disease. In regard to metastatic epidural spinal cord compression (MESCC), data from the 1970s from Gilbert and colleagues1 and from Greenberg and colleagues2 showed that a significant proportion of patients entering the hospital in an ambulatory state left with varying degrees of paresis and paralysis. Specialists from that era emphasized the need for earlier diagnosis and improved treatment.3 Unfortunately, proposed diagnostic algorithms relied heavily on the only available diagnostic tool at the time: myelography.4 Early opposition existed for use of such an invasive test on neurologically intact patients with cancer who had new-onset back pain; however, Rodichok and colleagues4,5 demonstrated that roughly 60% of patients with new back pain, abnormal findings on a plain spinal radiograph, and normal findings at neurologic examination who underwent myelography were shown to have epidural cord compression. Since that time, diagnosing spinal cord compression before clinically apparent neurologic dysfunction has remained paramount in the management of metastatic vertebral disease. Early diagnosis and early, aggressive treatment are the hallmarks of current treatment. Currently, approximately 30% of patients with cancer experience symptomatic spinal metastases during the course of their illness, and as many as 90% of patients with cancer have metastatic lesions within the spine at the time of death.6 With advances in the treatment of systemic oncologic disease, patient survival rates have increased during the past few decades. This increase, combined with improved imaging modalities of the neurologic and musculoskeletal systems (e.g., magnetic resonance imaging [MRI] and bone scintigraphy), will undoubtedly increase the incidence of spinal metastases encountered by physicians. As a result, it is essential that persons with cancer and those caring for them understand not only the myriad ways in which spinal metastases present but also the means by which they are currently diagnosed and managed. In this chapter, we attempt to address these issues by using an evidence-based approach from the most recently compiled literature. In addition, we propose a simple algorithm to aid in deciding which patients are best treated with medication and which patients could benefit from surgery. More than 1.4 million new cases of cancer are diagnosed annually in the United States,7 and roughly 500,000 persons die annually from metastatic disease.8 Metastases to the skeletal system occur third in frequency behind metastases to the lungs and liver.9,10 Within the skeletal system, the spinal column is the most commonly affected site. Cadaver studies have shown that as many as 30% to 90% of patients with terminal cancer have metastatic disease to the spine.11–15 Although it is estimated that only roughly 10% of patients with cancer experience symptomatic spinal metastasis,16 the prevalence of spinal metastases is likely to increase as duration or length of survival duration for many patients with cancer continues to improve. The highest incidence of clinically detected spinal metastases occurs during midlife (40 to 65 years of age), which corresponds to the period of increased cancer risk.17 Primary breast, lung, and prostate cancers represent the most common histologies that are metastatic to the spine, reflecting both their higher prevalences and their tendencies to metastasize to bone.18,19 A slightly higher incidence in men is related to the slightly higher incidence of prostate cancer compared with breast cancer.17 However, as adjuvant treatment for breast cancer improves overall survival duration, this dissimilarity could vanish. Figure 49-1 shows the relevant anatomy of the spinal cord, spine, and associated structures and the location of metastatic lesions in these areas. Lesions that cause spinal cord compression usually first invade the epidural space, most often as direct extension of metastatic disease from the vertebral body. Spine metastases are thought to arise by various mechanisms, often predicated on the biological behavior of the primary cancer. • Hematogenous spread. As the most common means of malignant tumor spread to the skeleton, hematogenous spread likely occurs by both venous and arterial routes. Batson’s venous plexus is the longitudinal network of valveless veins that course parallel and juxtaposed to the spinal column. This plexus communicates with multiple venous systems (e.g., the spine, vena cava, portal, azygous, intercostals, pulmonary, and renal), and the flow direction within the plexus may be variable because of changes in intrathoracic and intraabdominal pressures. In this way, tumors in multiple sites of the major body cavities could deposit tumor cells in the spine.20 Because of the significant blood flow to the vertebral bodies, the arterial system also is effectively able to deliver tumor cells to these well-perfused bones.21 Although hematogenous dissemination of tumor cells is less common, it may also lead to direct metastasis to the cord (intramedullary) or to the epidural space itself. • Direct extension. Primary tumors located in paravertebral soft tissues can often extend into the vertebral column via intervertebral foramina. Lung cancer, for instance, may become locally aggressive and invade the thoracic spine, whereas prostate, bladder, and colon cancer may invade the lumbar and sacral spine.20 • Cerebrospinal fluid spread. Cerebrospinal fluid spread is usually conferred by “shedding” of tumor cells from cerebral or cerebellar metastatic lesions, often after surgical manipulation of brain metastases.22 After a malignant lesion has begun to grow in the epidural space, it is unclear how or at what point spinal cord compression (as seen on MRI or myelogram) leads to clinically apparent spinal cord compromise. Animal models demonstrate that at least three mechanisms may be at play.25–25 First, direct pressure on the neural structures of the cord may result in demyelination. Second, compression may lead to arterial insufficiency with subsequent neural degeneration. Finally, compression may cause venous blockage with secondary vasogenic edema as a result of disruption of the blood–spinal cord barrier. Interestingly, results of various animal experiments have shown that the spinal cord can tolerate prolonged pressure if the onset of the pressure is slow and the neural degeneration is not irreversible.28–28 Along these lines, Rades and colleagues found that slower clinical progression of neurologic symptoms before diagnosis led to improved outcome,29 emphasizing again that early diagnosis is a key to successful management. Significant back pain will likely affect more than 80% of Americans at some point in their lives.30 Because the overwhelming majority of such patients have noncancerous musculoskeletal pain, the presenting complaint of back pain may lead many physicians to ignore these complaints from patients who will ultimately harbor underlying spinal metastatic disease. However, in roughly 10% of patients with developing cancer, symptomatic spinal metastases may be the initial presentation.31 In addition, when vertebral lesions are discovered, pain is the most common presenting symptom, occurring in approximately 83% to 95% of patients.3,18,32 As a result, a patient with cancer who reports back strain from a particular event still needs very close attention and follow-up care.33 Three classic pain syndromes affect patients with spinal metastases: local, mechanical, and radicular pain. Patients often present with a combination of these pain syndromes. Local pain is usually described by patients as a persistent “gnawing” or “aching” pain emanating from the region of the spine that is affected by metastatic disease. It is hypothesized that growth of the metastatic tumor, most commonly located in the posterior vertebral body, leads to periosteal stretching and/or a local inflammatory process that stimulates the pain fibers within the spinal periosteum. In such patients, percussion over the spinous process may elicit local tenderness. Such pain usually responds well to steroid administration.34 Mechanical pain, also known as axial back pain, is aggravated by movement, activity, or simply increasing weight-bearing forces on the spinal segment affected. Spinal metastases that result in vertebral body damage (e.g., deformity or fracture) may result in spinal instability, which likely results in muscle, tendon, ligament and/or joint capsule strain and ensuing symptoms of mechanical pain. Unfortunately, such discomfort is usually refractory to narcotics and steroids but responds to recumbency, bracing, and/or internal stabilization. Radicular pain may occur when spinal lesions compress or irritate an exiting nerve root, yielding pain in the dermatomal distribution of the involved root that is often described as “sharp,” “shooting,” or “stabbing.” Interestingly, dysesthetic/neuropathic pain may also arise when patients have intradural extramedullary disease, creating pain that may be described as an “intense, burning” sensation.22 The second most common presenting complaint of patients with vertebral metastases is motor dysfunction, which can manifest as myelopathy and/or radiculopathy. Although it is less common than the presentation of pain alone, roughly 60% to 85% of patients with MESCC are weak at the time of diagnosis.2,3,18 In addition, patients often have some level of bladder, bowel, and/or sexual dysfunction related to the MESCC that they will not readily report to the physician unless an inquiry is made. Bladder dysfunction is the most common autonomic finding and commonly correlates with the degree of motor dysfunction.8 Although the rate of clinical progression is variable, patients with motor dysfunction inevitably progress to complete paralysis in the absence of intervention.35 In addition, severe autonomic dysfunction with urinary retention, constipation, and loss of control of bowel or bladder function is a late and particularly ominous finding because full paraplegia can follow within hours.33 Neurologic status at the time of diagnosis, particularly motor function, has been shown to correlate with prognosis from MESCC,21,36 thus reinforcing the concept that diagnosis before the development of a neurologic deficit is of paramount importance. Unfortunately, because complaints of chronic back pain in the general population are extremely common,30 it is likely that a delay in diagnosis of vertebral metastasis occurs frequently in patients who report merely new-onset back or neck pain. In a study of 319 patients with cancer, Levack and colleagues37 reported that a median of 2 months passed from the onset of pain, as reported to their primary care providers, until the diagnosis of metastatic spinal cord compression. For this reason, new-onset back or neck pain in a patient with known cancer must be considered to be spinal metastatic disease until proven otherwise. Moreover, thoracic pain is less common than is pain originating from the mobile cervical and lumbar regions, where degenerative disease is the more common precipitating cause of pain; thus pain in the thoracic region should raise a high level of suspicion for the likelihood of cancer. In patients with known or suspected malignancy who have back pain, the first step is a neurologic examination (Fig. 49-2). If the presence of an abnormal finding is in question, assistance should be sought from a neurologist. Any sign of myelopathy should lead to a prompt MRI of the entire spine with institution of high-dose dexamethasone therapy before the patient is sent to the MRI suite. Because a single bolus of steroid is unlikely to cause any significant adverse effects, waiting for confirmation of spinal cord compression before instituting administration of steroids is almost never warranted.38 Plain radiographs have long been the first course of imaging obtained for patients with spine pain, mainly because of ease of use and relatively low cost. They serve mainly as a screening test by revealing lytic or sclerotic areas of bone, pathological compression fractures, deformity, and/or paraspinal masses. The major proportion of spinal metastatic lesions are osteolytic, but as much as 50% of the bone must be eroded before there is a noticeable change on plain radiographs.39 Notably, osteoblastic/sclerotic lesions most often result from carcinoma of the breast or prostate.40 Bone scanning or nuclear scintigraphy is sensitive for identifying increased metabolic activity throughout the entire skeletal system. Thus whereas plain films might not detect tumor-induced radiographic changes until 30% to 50% or more of the vertebral medullary space has been replaced,41 bone scans may reveal the lesion at an earlier stage.42 However, such scans may detect increased metabolic activity associated with spinal inflammation and infection, leading to overall decreased specificity. In addition, nuclear scintigraphy currently has poor imaging resolution, necessitating correlation with CT or MRI to exclude benign processes and to plan possible operative intervention.43 PET scanning with 18F-fluorodeoxyglucose is now more commonly used for whole-body metastatic surveys and as a staging technique in patients with known systemic cancer.44 However, as with nuclear scintigraphy, poor spatial resolution in PET necessitates concomitant use of CT or MRI. MRI is considered the gold standard imaging modality for assessing spinal metastatic disease.47–47 MRI is more sensitive than standard radiographs, CT, and bone scans in detecting primary malignant bone tumors and metastatic lesions in the spine.48,49 Such sensitivity is due to the fact that MRI allows for superior resolution of soft-tissue structures such as intervertebral discs, spinal cord and nerve roots, meninges, and paraspinal musculature. Moreover, MRI provides clarity at the osseous–soft-tissue interface, yielding accurate anatomic detail of bony compression or invasion of neural and paraspinal structures. When a patient has either local or radicular back pain with no evidence of myelopathy, a scanning MRI should be ordered.50 This study involves a series of T1-weighted sagittal images extending from the cervical spine to the sacrum. If metastatic lesions of bone are found, the procedure is converted to a more comprehensive set of images. This method is a faster and more cost-effective approach to early diagnosis of spinal cord compression.50 The comprehensive MRI protocol should include T1- and T2-weighted images and contrast-enhanced studies that provide axial, sagittal, and coronal reconstructions.51 Because of the high signal intensity of fat within bone marrow on T1-weighted images, fat suppression techniques are useful in evaluating osseous lesions that enhance with contrast material.51 Diffusion-weighted imaging may be helpful in distinguishing between benign and pathological compression fractures.52 Angiography is an advanced imaging modality that is ordered by the neuroradiologist and/or neurosurgeon once the diagnosis of a spine tumor has been made. In patients with spinal metastases that are suspected to result from highly vascular primary tumors, such as renal cell carcinoma, thyroid carcinoma, angiosarcoma, leiomyosarcoma, hepatocellular carcinoma, and neuroendocrine tumors (e.g., pheochromocytoma and paraganglioma),53 angiography may provide diagnostic information and have therapeutic benefit. If the lesions are considered for surgery, preoperative knowledge of the vascular supply may prove invaluable. In addition, angiography might allow preoperative embolization of the spinal lesion, leading to decreased blood loss with operative resection.54 Such intraoperative blood loss can influence the surgeon’s ability to visualize an adequate view of the surgical field, thus affecting achievement of complete resection. In addition, significant blood loss may be associated with life-threatening hemorrhage intraoperatively, longer operating times, intraoperative complications, postoperative hematomas, and wound breakdown. In patients who are not candidates for surgery, embolization may be effective as primary treatment for the lesion.55 Although imaging modalities have improved vastly during the past two decades, acquisition of tissue is not uncommonly needed for diagnosis. If surgery is not indicated up front and no other systemic lesions can be found, percutaneous image-guided biopsy may be indicated. Improved CT fluoroscopy and needle biopsy systems provide relatively easy access to most lesions, with success rates approaching 90%. The majority of such procedures are now performed in the outpatient setting.56,57 Treatment of spinal metastases is primarily palliative. As a result, goals of treatment are centered on pain relief, maintenance or restoration of spinal stability, and preservation of neurologic function. Although patients may be cured with radical surgical excision in rare circumstances (e.g., solitary renal cell carcinoma spinal metastasis),58 patient variables, including age, tumor burden, life expectancy, and functional status, overwhelmingly influence the choice of therapeutic options. The three traditional mainstays of therapy have been corticosteroids, radiation therapy, and surgery.59 In the following paragraphs, we will provide the most current treatment paradigms involving each of these modalities and describe how technologic advances (e.g., bisphosphonates, radiosurgery, percutaneous vertebroplasty, and aggressive decompressive surgery) have increased the armamentarium against metastatic spine disease and have consequently improved outcomes (Fig. 49-3). Chemotherapeutic agents can be classified into antitumor drugs and drugs that minimize the secondary effects of the tumor.60 Except in cases of chemosensitive tumors, such as Ewing sarcoma and neuroblastoma, antitumor drugs continue to have a limited role in the treatment of spinal metastases. On the other hand, drugs that are used to prevent or ameliorate the effects of spinal tumors (e.g., corticosteroids, bisphosphonates, and analgesics) are widely accepted. The three most common primary tumors that metastasize to the spine are breast, prostate, and lung cancers.18,19 Unfortunately, although lung cancer is still the leading cause of cancer mortality in the United States, the median survival of patients with lung cancer metastatic to bone is reportedly between 3 and 9 months. As a result, most patients die of progressive disease before skeletal complications become a large problem.60 In most prostate and breast cancers, on the other hand, lesions may be sensitive to hormonal manipulation.60,62 Estrogen agonists/antagonists, such as tamoxifen, and aromatase inhibitors, such as letrozole, have been shown to be effective for breast cancer. For prostate cancer, androgen deprivation is the mainstay of treatment with GnRH agonists and/or flutamide.
Spinal Cord Compression
Introduction
Epidemiology
Pathophysiology
Clinical Evaluation
Back Pain
Motor and Autonomic Dysfunction
Diagnosis
Plain Films
Bone Scan
Magnetic Resonance Imaging
Angiography
Percutaneous Spine Biopsy
Treatment
Hormonal Therapy, Chemotherapy, and Medical Therapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Spinal Cord Compression