40 Spinal Column Tumors
Spinal tumors arise from all tissue types from which neoplastic changes could occur, including neural element, meninge, bone, cartilage, and muscle. This chapter addresses the clinical presentation, diagnosis, management, and outcomes of primary and secondary osseous neoplasms affecting the spine. Advances in evidence-based medicine and treatment options, especially with regard to surgical interventions, have initiated a paradigm shift in the management of both primary tumors, where treatment aims to cure, and secondary tumors, where surgery is now not only a palliative measure but also has increasingly been used for improving functional outcomes and quality of life.
 Primary Tumors
Primary Tumors
Primary spinal tumors are rare, accounting for less than 5% of osseous tumors and 0.2% of all cancers.1 They are classified as benign (Table 40.1) and malignant (Table 40.2). Both benign and malignant tumors may be associated with spinal instability and deformity (Table 40.3). They also typically present as persistent local back pain, often occurring at night. Age and location provide provide central clues to help physician distinguish between spinal benign and malignant tumors. While over 65% of the pediatric spinal tumors are benign, the same proportion of lesion is malignant in adults. In addition, 75% of the neoplasms located in the vertebral body are malignant, but 65% of those involving the posterior elements are benign.2
Benign spinal tumors can be difficult to diagnose; they may be incidental findings, and they tend to affect young adults. Although they can be associated with radiating radicular symptoms in the lower extremities, they seldom cause focal neurologic deficits as a result of spinal cord or nerve root compression.
Malignant osseous tumors commonly present with unremitting axial back pain that often does not respond well to analgesics. They normally affect older adults, tend to progress more rapidly, and recur more often. They are also associated with higher morbidity, including more risk of neurologic deficits and mortality. In fact, they have a greater propensity (1) to create pathological fractures; (2) invade and destroy adjacent structures, which can compromise the spinal cord, nerves, or blood vessels; and (3) metastasize to other organs.
Clinical evaluation of primary spinal tumors includes a thorough history and physical examination and adequate imaging [including plain X-rays, computed tomography (CT), and magnetic resonance imaging (MRI)]. CT-guided needle biopsy is often indicated to guide therapy, and bone scans may be required to search for other bone lesions. CT of the spine is the best modality to characterize bony involvement, quality, and anatomy, which can be very valuable for surgical planning.6
Pearls
• Osteoid osteoma/osteoblastoma presents as painful scoliosis in about 50% of children or young adults, which typically disappears after the tumor is completely surgically excised.
• Serum protein and immune electrophoresis should be done in all adult patients with pathological vertebral compression fracture with no obvious primary malignancy to rule out multiple myeloma/plasmocytoma.
Staging
Enneking and Weinstein-Boriani-Biagini (WBB) classification systems have been used for primary spinal tumors. Boriani et al modified the original Enneking staging system to classify benign and malignant primary spinal tumors. This classification divides benign tumors into three stages (SI, SII, and SIII) and malignant spinal tumors into six stages (IA, IB, IIA, IIB, IIIA, and IIIB) based on surgical grade (G, G1, G2), local extent (T, T1, T2), and presence or absence of metastasis (M0, M1) (Table 40.4). The stage of the tumor dictates the extent of surgical resection and margins.2–5
Primary benign spinal tumors in the SI (latent/inactive) stage are either not growing or doing so extremely slowly; have a true capsule, and thus well-defined margins; and are generally asymptomatic. These tumors include incidental vertebral body hemangioma, occurring in about 10% of the population. Unless palliative intracapsular excision is required for decompression or stabilization, SI tumors are typically managed conservatively, with reassurance and observation.2,5
SII benign lesions (active) are bordered with a thin/pseudocapsule surrounded by a layer of host reactive tissue and confined to the vertebral osseous elements; bone scans are often positive. They are slow-growing tumors and cause mild symptoms. Benign osteoblastomas may present as such and typically appear as a radiolucent nidus (> 20 mm) surrounded by a sclerotic rim and located in the posterior elements of the spine. They are generally managed by intralesional excision or curettage with low reported recurrence rates.2,5
Table 40.1 Benign Primary Spinal Tumors (alphabetical order)
| Tumor Type | Characteristics |
| Aneurysmal bone cyst | Consist of blood-filled cavity; often occur in patients in 20s; slow, gradual onset of back pain; possible palpable mass; lytic and expansile lesions in thoracolumbar area often in posterior elements, in multiple continuous levels |
| Eosinophilic granuloma | Results from Langerhans’ cell histiocytosis; usually males < 10 years old; more often in vertebral body of thoracic spine; may be associated with systemic involvement (Hand-Schüller-Christian or Letterer-Siwe disease) |
| Giant cell tumor | Most often in vertebral body; 20–50 years of age; progressive back pain; expansile, radiolucent, “soap-bubble” appearance; vascular; often locally aggressive with bony destruction; en bloc excision with wide margins |
| Hemangioma | Most common benign spinal tumor; Incidence increases with age; Multiple ~ 30% of the time; Typically incidental finding ~10% of patients; Often asymptomatic vascular intraosseous lesions in thoracolumbar area |
| Neurofibroma | Derived from Schwann cells; most frequent in cervical and thoracic areas; occur in isolation or associated with neurofibromatosis; dumbbell-shaped |
| Osteoblastoma | Similar to osteoid osteoma, but larger diameter (> 20 mm), histologically, and clinically more aggressive; surgically managed with en bloc resection |
| Osteochondroma | Most frequent in posterior element; often in cervical area (C2), because of osteocartilaginous proliferation at a growth plate |
| Osteoid osteoma | Small bone-forming tumor (15–20 mm); ~10% primary bone tumors; ~10% occur in vertebrae, primarily in posterior elements; progressive back pain, typically at night; males more affected than females; most common cause painful scoliosis in adolescent population; radiolucent nidus with sclerotic rim; typically respond to anti-inflammatory medication; surgery is curative |
Table 40.2 Malignant Primary Spinal Tumors (alphabetical order)
| Tumor Type | Characteristics |
| Chondrosarcoma | Often males in 40s; tumor divided into low, intermediate, and high grades; destructive lesion often with paraspinal calcified mass causing axial pain with neurologic deficits |
| Chordoma | Often slow growing, locally invasive, not likely to metastasize; > 40 years old; often cranial or sacral origin; median survival is 50 months |
| Ewing’s sarcoma | Typically males in 20s; often lytic, sacral, poorly delineated from normal bone lesion that invades surrounding tissues; chemotherapy ± radiation |
| Lymphoma | “Epidural lymphoma,” which is a tumor within the epidural space that can compress the spinal cord without obvious bony involvement; treatment involves chemotherapy |
| Malignant fibrous histiocytoma | Osteolytic destruction of vertebrae and invasion of paraspinal structures at multiple levels, can cause neurologic deficits; tends to recur and metastasize |
| Osteosarcoma | Peak occurrence during onset of puberty; mostly osteoblastic; when metastasis present, most often to lungs; 18% survival rate at 5 years |
| Plasmocytoma/multiple myeloma | Most frequent primary malignant lesion of spine; osteolytic bone lesion(s) or diffuse osteoporosis ± fractures; high serum proteins and Bence Jones proteins in urine, renal dysfunction, high erythrocyte sedimentation rate |
Table 40.3 Clinical Features of Benign and Malignant Primary Spinal Tumors
| Clinical Features | Benign | Malignant |
| Age | Young adults (20–30 years) | Middle-aged adults (40–60 years) |
| Axial spinal pain, often occurring at night | Common (75%) Common (95%) | Severity progresses more rapidly than with benign tumors |
| Neurological deficits | 20% | 55% |
| Scoliosis/Spinal deformity | May occur | May occur |
| Vertebral body involvement | 40% | 80% |
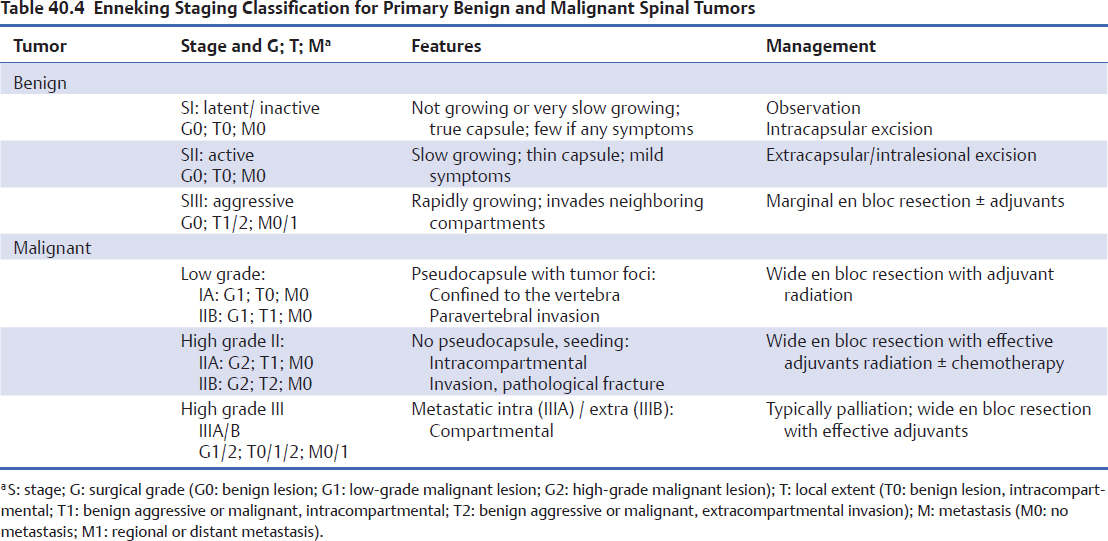
SIII tumors (aggressive) have a very thin, incomplete, or absent capsule, often associated with a reactive and hyper-vascularized pseudocapsule. They are rapidly growing and invade neighboring compartments. Bone scans on this type of tumor are positive. Giant cell tumors (Fig. 40.1) and aneurysmal bone cysts are examples of these lesions. Management involves aggressive marginal en bloc resection. Adjuvant treatments, such as preoperative embolization, are often needed.2,5 The benefits of adjuvant radiation in cases of incomplete re-section and local recurrence or to prevent delayed local recurrence must be weighed against the risk of developing radiation-induced myelopathy, spinal deformity, and malignant conversion (sarcomatous degeneration).6
Low-grade malignant spinal tumors (I) are subdivided into those that are confined to the vertebra (stage IA) and those that invade paravertebral compartments (stage IB). They have no true capsule, but a thick pseudocapsule of reactive tissue containing islands of tumor tissue. Despite safe optimal wide en bloc resection, which is sometimes not feasible, residual active tumor foci might be left behind; thus, postoperative radiotherapy is recommended.2,5
Special Consideration
• In primary spine tumors, the Enneking and Weinstein-Boriani-Biagini staging classification systems are very useful in assisting surgical staging and operative planning.
High-grade stage IIA (intracompartmental confined) and IIB (spread beyond the vertebral compartment) have such rapid growth that there is no host-reactive tissue forming around the lesion; thus, there is no pseudocapsule forming. These tumors are constantly seeding with neoplastic nodules (satellites); they are often associated with neoplastic nodules occurring at some distance from the main tumor mass (skip metastases). When those neoplastic seedings create distant metastasis, they are stage IIIA (intracompartmental confined lesion of origin) and IIIB (extracompartmental lesion of origin). These radiolucent high-grade lesions are often associated with pathological fractures and extensive invasion of adjacent compartments, such as the epidural space, leading to important neurologic deficits. High-grade stage II tumors are managed with wide en bloc resection and adjuvant therapies, including radiation and chemotherapy based on the histological type of the tumor, for local control and prevention of distant spread.2,5 A recent study reported that patients who underwent a surgical resection for a primary chordoma, chondrosarcoma, Ewing’s sarcoma, or osteosarcoma had longer overall survival independently of patient age, extent of local spread, or location.7 High-grade stage III tumors are typically treated palliatively.5
The Enneking staging system has several limitations. Because it was originally based on the natural evolution of mesenchymal musculoskeletal appendicular tumors, it is not applicable to tumors originating in the marrow or reticuloendothelial system, including lymphoma, multiple myeloma/plasmocytoma, Ewing’s sarcoma, and other round cell neoplasms. Also, the Enneking classification does not take into account the dimension of the primary tumor. The size of a tumor is often a significant prognostic factor, with larger lesions being more likely to metastasize. Lastly, this system does not consider (1) the presence of the epidural space as a continuous compartment, (2) the need for maintaining or restoring spinal stability, and (3) the close proximity of crucial structures, notably the spinal cord and nerve roots, which, if sacrificed, lead to important neurologic deficits; consequently, honoring Enneking surgical margins can be difficult.3–5
• Given the potential to injure the spinal cord or nerve roots, it is sometimes difficult to respect Enneking surgical margins.
Treatment
Surgery
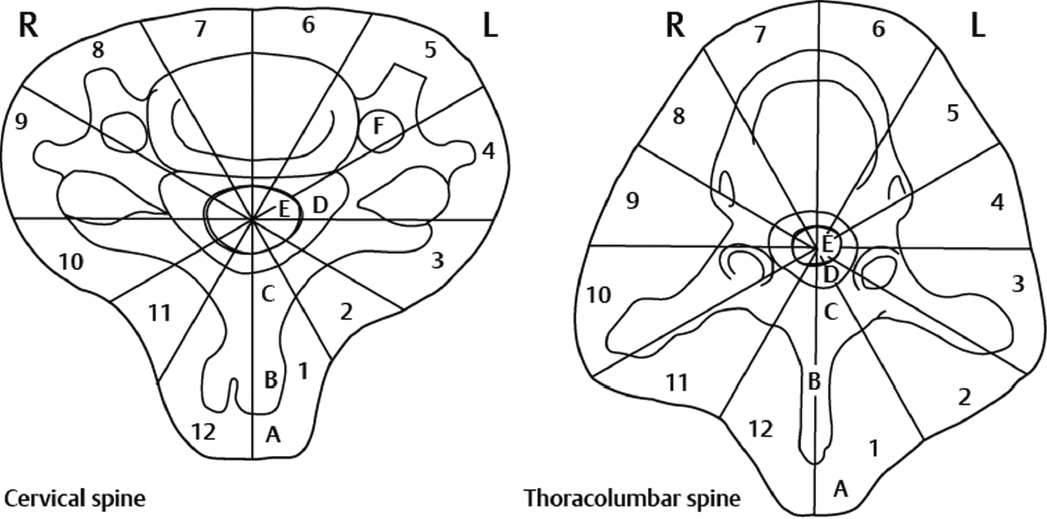
The WBB classification was developed to assist decision making with respect to feasible surgical margins in spinal oncological surgery. In this system, the involvement of the vertebra, neural canal, and surrounding soft tissues are determined based on the axial plane, in terms of 12 radiating zones numbered 1 to 12 in a clockwise fashion and of five concentric tissue layers identified as A to E from the paravertebral extraosseous region to the dura, which relate to the compartments of tissue penetration involved (Fig. 40.2). The longitudinal extent of the tumor is identified as the number of vertebrae involved. The WBB system promotes optimal surgical tumor margins without violating the spinal cord. Therefore, it is a helpful surgical planning tool which helps determine the type of resection as well as its feasibility.8
In primary spinal tumors, the Enneking and WBB staging classification systems are key tools that assist with surgical staging and operative planning. Moreover, these classification systems show near-perfect intraobserver and moderate interobserver reliability.3,5 Due to the low incidence of primary osseous spine tumors and consequently the lack of clinical experience and high-quality evidence-based research, there is no validated oncological treatment management protocol. Fisher et al9 reported that using the surgical principles of the Enneking classification, originally introduced for the management of appendicular musculoskeletal tumors, is associated with a significant decrease in local recurrence and mortality. Indeed, the Enneking system helps avoid entering the tumor and an intralesional or piecemeal removal. Thus, it favors removing the tumor as one whole piece (en bloc) with a margin along the pseudocapsule of the tumor (marginal resection) or with a margin of normal tissue around the tumor (wide resection).9
Because residual tumor tissue is the single most important predictor of local recurrence, the goal of marginal or wide en bloc resection is to prevent tumor cell contamination during surgical removal of the primary tumor and ensure total tumor resection.10 It has been suggested that better local control, prognosis, and quality of life justifies marginal or wide en bloc resection in the management of aggressive benign and low-grade malignant primary spine tumors in spite of the associated significant morbidity.11 Indeed, spinal en bloc resections may involve sacrificing relevant structures such as the nerve roots and blood vessels, thus increasing the morbidity and compromising neurologic function.
Fig. 40.2 The Weinstein-Boriani-Biagini surgical staging system. On the axial plane of the cervical and thoracolumbar spine, the radiating zones numbered 1 to 12 identify the transverse extension of the vertebral lesion, and the five concentric layers A to E described the paravertebral extraosseous compartments to the dural involvement; in the cervical spine, the layer F indicates the involvement of the vertebral artery. The number of vertebral bodies involved determined the longitudinal extension of the tumor.
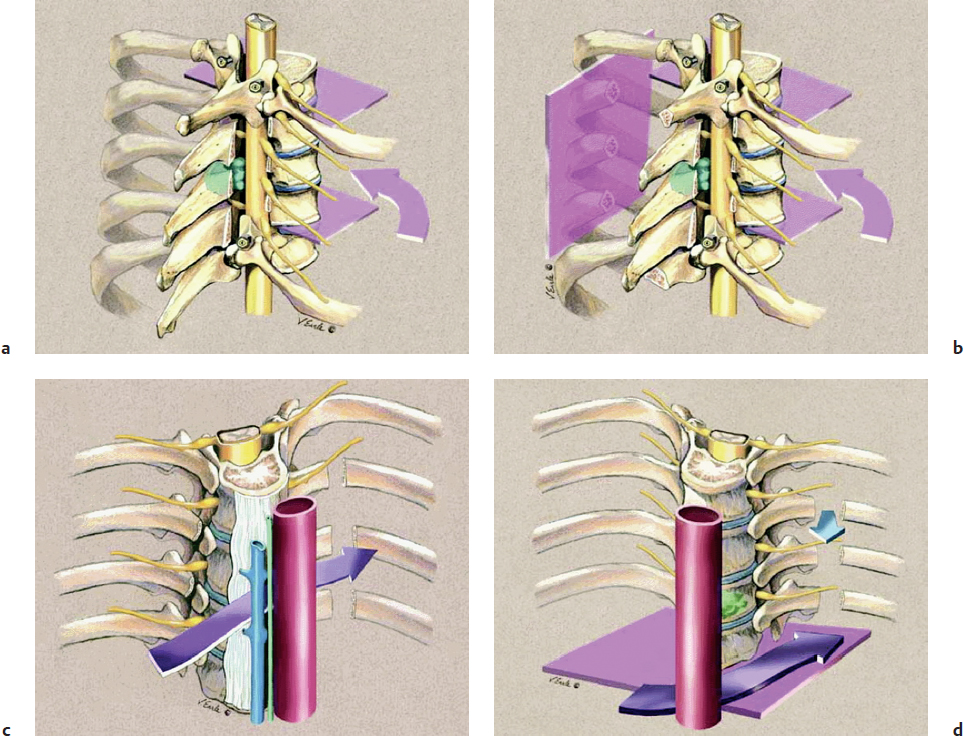
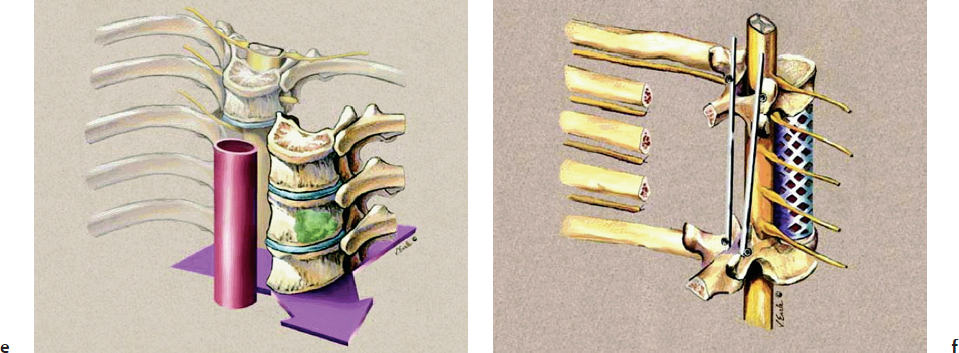
Multiple surgical techniques have been described in the literature to achieve en bloc resection in primary osseous spinal neoplasms. A staged approach has been described as an excellent option (Fig. 40.3).12 The initial stage implies placing posterior spinal instrumentation and performing laminectomies, rib head resections, corpectomies, and diskectomies in order to isolate the tumor from the spine. Then, the second stage involves the marginal or wide en bloc resection encasing the tumor using a combined anterior and posterior approach, followed by reconstruction and stabilization of the anterior spinal column.
Pitfall
• Spinal oncological en bloc resections are high-risk and demanding interventions that should be performed in properly equipped centers by experienced surgeons.
Spinal oncological en bloc resections are high-risk and demanding interventions, which should be performed in tertiary care centers by experienced surgeons. In addition, patients should be evaluated by a multidisciplinary team involving spine surgeons, radiologists, radiation and medical oncologists, and pathologists. Management decisions should include careful consideration of tumor size, histology, neurologic deficits, patient’s age and medical status, as well as realistic long-term goals and expectations. Increasing age, the presence of metastasis, and more extensive tumor invasion have been identified as independent factors associated with poor survival in patients with primary malignant osseous tumors, specifically osteosarcomas, chondrosarcomas, and chordomas.13 McGirt et al13 have proposed a five-grade system in which higher scores are closely correlated with decreased survival (Table 40.5). Although this system is still pending validation, it could potentially assist with survival prognostication and consequently guide the aggressiveness of the treatment strategy proposed.
Stereotactic Radiosurgery
Stereotactic radiosurgery (SRS) entails radiating a highly specific target volume under image guidance with a high dose per fraction, sparing adjacent nontumor tissue. Its usage in the treatment of intracranial and extracranial tumors is blooming as an adjunct to surgery, fractioned radiotherapy, and chemotherapy, as well as being a primary treatment option, with good target localization and delineation from adjacent structures. The well-established role of SRS in the treatment of intracranial metastatic lesions is now extended to both primary and metastatic spinal osseous neoplasms, optimizing local control and pain relief.14–16
Although excisional surgery is the mainstay treatment of primary spinal bone tumors, open surgery can be difficult or risky to perform in some patients due to advanced age, medical comorbidities, anatomic location or multiple locations of the tumor, recurrent tumor after an open resection, or location in an area to which external beam radiation has already been applied. Therefore, patients with well-circumscribed lesions, relatively little direct compression on the spinal cord, lesions not requiring open surgery for spinal stabilization, and relatively short life expectancy might benefit from SRS.14–16
Patient Selection for Stereotactic Radiosurgery
• Well-circumscribed lesions
• Minimal spinal cord compromise
• Previously irradiated lesions precluding further external beam irradiation
• Recurrent surgical lesions
• Lesions requiring difficult surgical approaches
• Relatively short life expectancy as an exclusion criterion for open surgical intervention
• Significant medical comorbidities precluding open surgical intervention
• Lesions not requiring open spinal stabilization techniques
(Modified with permission from Gerszten PC, Ozhasoglu C, Burton S, et al. CyberKnife frameless stereotactic radiosurgery for spinal lesions: clinical experience in 125 cases. Neurosurgery 2004;55:89–98.)
 Metastatic Tumors
Metastatic Tumors
Metastatic osseous tumors are by far the most common tumor affecting the spinal column; they are about 25 times more frequent than primary bone cancers.17 Aging of the population and advances in diagnostic and treatment options are increasing the number of primary cancer survivors; indeed, both the prevalence and the incidence of cancer are increasing.18 Consequently, the incidence of metastatic spine cancer is also expected to rise.
Although metastatic bone disease rarely causes death directly, it is associated with shorter life expectancy and significant morbidity, such as pain, fractures, hypercalcemia, and neurologic deficits resulting from spinal cord or nerve root compression and damage.17,19,20 Consequently, quality of life may be dramatically affected. Primary tumor type influences the patient’s prognosis. The median survival in patients with bone metastases ranges from a few months for lung cancer to several years for breast or prostate cancer.17,20 Metastatic epidural spinal cord compression (MESCC) is an oncological emergency that requires early diagnosis and expedited treatment.
Epidemiology
After the lungs and the liver, bones are the third most common site for cancer metastasis, with the spinal column being the most frequent skeletal location affected.17,19 Not all bone metastases are clinically significant; 30 to 90% of patients who die from cancer are found to have bone metastasis at autopsy.19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree