Jorge J. Castillo and Tina Rizack • Cancer complicates 1 in 1000 pregnancies; the most common malignancies in pregnant women are breast, uterine, and cervical cancer, lymphoma, and melanoma. • No evidence exists that pregnancy alters the clinical behavior of cancer, but cancer often is advanced at diagnosis because of the overlap of symptoms with those of a normal pregnancy. • Important factors in management include assessment of gestational age, maternal staging with limited exposure to ionizing radiation, the urgency of therapy, and the impact of therapy on maternal prognosis and fetal outcome. • Physiological changes in pregnancy affect the metabolism of chemotherapy drugs, but few practical guidelines exist about how dosing should be adjusted to take this factor into account. • Alkylators and antimetabolites should be avoided in the first trimester, but these agents and other cytotoxic drugs are generally not contraindicated (with the possible exceptions of methotrexate and hydroxyurea) in the second and third trimesters. • The scheduling of chemotherapy should be planned to minimize the risk of complications at the time of delivery. • No evidence exists that exposure to chemotherapy results in long-term adverse effects on physical or intellectual development, and surviving children have no increased risk of malignancy. • Therapeutic radiation jeopardizes fetal outcome and should be reserved for the postpartum period whenever possible. • Subsequent pregnancy after cancer diagnosis and treatment is usually possible. This chapter focuses on the issues related to the care of women with cancer that is diagnosed during pregnancy. Cancer is the second leading cause of death in women between the ages of 20 and 39 years and complicates 1 in 1000 pregnancies. The most common cancers diagnosed in pregnant patients are the same as those seen in nonpregnant women of similar age: breast, uterine, and cervical cancer, lymphoma, and melanoma.1 Although pregnancy is associated with immunologic tolerance, no evidence exists of an increased incidence of cancer or of more aggressive behavior of malignancies that are diagnosed during pregnancy. However, many cancers in pregnancy are diagnosed at an advanced stage, often because symptoms of the malignancy overlap with those that are experienced during a “normal” pregnancy. Because most chemotherapy drugs are uncharged, lipophilic, of low molecular weight, and minimally protein bound, they cross the placenta to the fetal circulation. The placenta is the primary portal of exit of waste products and toxins from the fetus. However, the metabolites are generally more polar than the parent compound, might not cross the placenta as easily, and hence may accumulate in fetal tissues or amniotic fluid. The fetal liver can metabolize drugs as early as 7 to 8 weeks of pregnancy, but the extent to which fetal liver and kidneys participate in drug elimination is minimal.2 Pregnancy induces a number of important physiological changes that cause significant alterations in the metabolism and efficacy of commonly used medications (Box 64-1). However, few data exist to guide physicians in adjustment of drug dosing (see the Chemotherapy Dosing section). Radiation can be divided into ionizing and nonionizing radiation. Ionizing radiation has the ability to penetrate tissue and damage cellular DNA, resulting in mutation and ultimately affecting the development and viability of the fetus. Numerous studies of radiation exposure after the atomic bomb detonations in Japan confirmed that this effect is dependent on radiation dose and stage of fetal development at the time of exposure (Table 64-1).3 Fetal abnormalities associated with exposure to excessive ionizing radiation include microcephaly, eye malformation, and growth retardation.4 The American College of Obstetricians and Gynecologists recommendations for imaging during pregnancy state that 5-cGy exposure to the fetus is not associated with any increased risk of fetal loss or birth defects.5 Radiation exposure is well below this amount for most procedures except for the maximum dose with computed tomography (CT) scanning of the abdomen and pelvis (Table 64-2). A more relevant concern is an increased risk of childhood cancer. After a gestational age of 3 to 4 weeks, the number of excess cancer cases (leukemia and solid tumors) up to age 15 years after radiation in utero is estimated to be 1 in 17,000 per 0.1 cGy. The baseline cancer risk in the first 15 years is about 1 in 650, and thus fetal doses of 2.5 cGy will approximately double the risk; however, this represents an excess lifetime fatal cancer risk of less than 0.5%. Table 64-1 Estimates of Threshold Doses for Effects After Fetal Radiation in Utero Table 64-2 Typical Ranges of Fetal Doses* After Common Diagnostic Procedures *The radiation doses have been estimated from surveys conducted in the United Kingdom for a range of diagnostic radiology. Ultrasound is believed to be safe during pregnancy and can be particularly useful in assessing the breasts and liver.6 CT scans and noncontrast magnetic resonance imaging (MRI) scans are being used with increasing frequency during pregnancy and lactation. Radiation doses from CT of the head and chest are minimal.7 CT should be used as the initial study for suspected pulmonary embolism. Noncontrast MRI does not expose the patient to ionizing radiation, and there has been no indication that noncontrast MRI during pregnancy has produced deleterious effects. Contrast agents such as gadolinium cross the placenta and are contraindicated. MRI of the abdomen is limited by motion artifact of the bowel. Pregnancy is a relative contraindication to positron emission tomography (PET) scanning with use of fluorine-18 fluorodeoxyglucose as a radiomarker, but termination is not generally recommended if a patient is found to be pregnant after undergoing a PET scan. A PET/CT scan has a representative dose of 8 mGy (PET) and 0.3 cGy (CT). The dose to the fetus may be higher because of close proximity of the mother’s bladder, where 18F-fluorodeoxyglucose is excreted. The U.S. Food and Drug Administration (FDA) has defined risk categories for all drugs based in part on the evidence in animals of fetal harm (Table 64-3). The majority of chemotherapeutic agents are category D, for which there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks. Extrapolation of teratogenic and mutagenic effects of chemotherapeutic agents from animals to human organogenesis is difficult, however, because of differences in susceptibility between species.8 The timing of fetal drug exposure is critical. Administration of drugs within 1 week of conception may result in a spontaneous abortion or a healthy fetus. During the first trimester, when organogenesis occurs, drugs may produce congenital malformations and/or result in spontaneous abortion. Other factors that may influence the probability of teratogenesis include the frequency of drug administration, duration of exposure, synergistic effects of multiple drugs, use of radiation, and individual genetic susceptibility. Table 64-3 Food and Drug Administration Risk Categories for Drugs Administered During Pregnancy Most human data about chemotherapy during pregnancy involve small series or case reports, which are prone to reporting bias. Specific or systemic information about the teratogenicity of individual cytotoxics or modern multiagent chemotherapy regimens is limited, particularly in the first trimester. Many reported malformations have occurred after exposure to multiple agents, making it difficult to assign blame to a single causative agent. It is important to note that the overall incidence of major congenital malformations with chemotherapy use after the first trimester is approximately 3% (close to the risk of the general population), although the incidence of minor malformations may be as high as 9%, and between 10% to 15% of all pregnancies result in a miscarriage or spontaneous abortion.9 Extrapolation from older data also might not be appropriate, because many of these drugs (e.g., alkylating agents such as nitrogen mustard and busulfan and antimetabolites such as aminopterin) are now rarely used. Limited experience in the first trimester with regimens such as Adriamycin (doxorubicin), bleomycin, vinblastine, and dacarbazine (ABVD) and cyclophosphamide, hydroxydaunorubicin (doxorubicin), Oncovin (vincristine), and prednisolone (CHOP) suggests low rates of teratogenicity.10 In the second and third trimesters, drugs very rarely cause significant malformations but could impair fetal growth and development. The current available literature suggests that chronic prenatal chemotherapy exposure does not result in learning or behavioral problems (also known as functional teratogenesis).10,11 Overall, the use of systemic antineoplastic therapy alone appears to be accompanied by significantly lower risk than is commonly appreciated. Table 64-4 details the available experience on the use of some of the more commonly used chemotherapy drugs in animals and at various stages of human pregnancy, together with the FDA category.12–37 The recommendations are those published by Briggs and colleagues,13 with some additional comments from the authors. The choice of chemotherapy agents should be based on the most current available literature. Table 64-4 Summary and Recommendations for Chemotherapy Drug Experience in Pregnancy Refer to the text for additional comments. Adapted from Briggs GG, Freeman RK, Yaffe ST. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. Methotrexate is widely distributed, including into fluid spaces such as amniotic fluid, and when given during the first trimester it is closely associated with fetal abnormalities, characterized by cranial dysostosis, hypertelorism, micrognathia, limb deformities, and mental retardation. In fact, methotrexate is commonly used in combination with misoprostol during the first trimester to medically induce abortions. However, methotrexate does not uniformly cause malformations, and a critical dose and timing may exist above which fetal malformations occur.24 Exposure to methotrexate in the latter trimesters has not been associated with significant malformations,13 but its elective use at this time, particularly in high doses, is still not recommended. 5-Fluorouracil (5-FU) was associated with multiple congenital malformations in the fetus of a patient who was found to be pregnant at week 14 after she began receiving chemotherapy for colon cancer at week 12 and hence is not recommended for use during the first trimester.38 Multiple studies have shown safe use beyond the first trimester in combination therapy.39,40 Use of cytosine arabinoside during the first trimester, alone and in combination with other drugs, has also been associated with congenital anomalies.36 Capecitabine is an oral drug akin to 5-FU that is used in persons with bowel and breast cancer. Capecitabine is embryotoxic in animals, is classified as a category D drug, and is not recommended for use during pregnancy. Use of hydroxyurea is believed to be unsafe during pregnancy because of a case series of 31 patients in which an increased risk of intrauterine growth retardation, intrauterine fetal demise, and prematurity was noted.41 Whether this finding was due to underlying disease is unclear. No data for cladribine or fludarabine exist in the literature. Alkylating agents are commonly used in the treatment of lymphoma, acute lymphocytic leukemia, multiple myeloma, ovarian cancer, and breast cancer. Cyclophosphamide is the most widely used and the best studied. Use in the first trimester has been associated with some fetal abnormalities, tempered with several reports with no abnormalities. Second and third trimester use is believed to be safe. Regarding dacarbazine use during pregnancy, congenital abnormalities were seen in two cases during the first trimester, consisting of isolated microphthalmos with secondary severe hypermetropia and a unilateral floating thumb malformation and one fetal death. In the second or third trimester exposure, one fetus died and one case of minor malformation (syndactyly) was observed. In all cases, dacarbazine was administered with several other cytotoxic drugs.42 Exposure to chlorambucil during the first trimester has been reported to cause renal aplasia, cleft palate, and skeletal abnormalities.43 Regarding cisplatin and carboplatin, a number of case reports documenting platinum use after the first trimester have not noted any congenital malformations.22,33 Oxaliplatin is embryotoxic in animals, is classified as a category D drug, and is not recommended for use during pregnancy, although one case has been reported without adverse outcomes.44 Although data are limited, the use of taxanes appears feasible and safe during pregnancy based on placental expression of drug-extruding transporters such as P-glycoprotein and BCRP-1, which result in low fetal exposure.45 Forty cases have been reported in the literature, including use of paclitaxel (21 cases), docetaxel (16 cases), and both drugs (3 cases).46 Ninety-five percent were treated after the first trimester and 95% received taxanes concomitantly with other chemotherapy. Despite the inherent bias in case reports, the pharmacokinetics of paclitaxel and docetaxel and available data suggest that these drugs appear safe for use in the second and third trimesters in cases where benefit is believed to outweigh risk. Although vinblastine is highly teratogenic in animal models, the literature suggests that its use in the first trimester may be relatively safe. In a series of patients treated with single agent vinblastine for Hodgkin lymphoma, no adverse outcomes to the pregnancy or in infants who were followed up afterward were reported.47 No congenital malformations were reported in 11 pregnancies when exposure to vincristine occurred, with exposure in three pregnancies occurring in the first trimester.19 Vinorelbine, vinblastine, and vincristine had been used during the latter trimesters without harm to the fetus.10,14 Little information is available about the effects of anthracyclines during pregnancy when they are used as single agents. Few malformations have been reported when anthracyclines are used in combination regimens in the first trimester. A few studies have shown acute myocardial dysfunction after anthracycline use in a minority of fetuses.48,49 However, a study of 81 children with in utero chemotherapy exposure, including anthracylines, who were followed up until age 9 to 29 years, showed that cardiac function did not differ from the general population.11 Idarubicin is more lipophilic, which favors placental transfer; it has been associated with neonatal cardiomyopathy, and its use during pregnancy is not recommended.32 Doxorubicin is preferred to daunorubicin and has been used in combination therapy in a prospective single-arm study of 57 pregnant women with breast cancer in the second or third trimester. No stillbirths, miscarriages, or perinatal deaths were observed.50 Similar findings have been reported in smaller series. Trastuzumab, a monoclonal antibody that binds to the extracellular domain of the human epidermal growth factor receptor 2 protein (HER2), is used in breast and gastric cancers. HER2 is strongly expressed in fetal renal epithelium. In a case series of 15 patients exposed to trastuzumab in utero, three fetuses had renal failure and four died. In addition, oligohydramnios or anhydramnios was present in eight pregnancies.51 Trastuzumab is not recommended for use during pregnancy. Bevacizumab, a humanized monoclonal antibody with an antiangiogenic effect, is used to treat multiple cancers. Because it is teratogenic in animals, consistent with a crucial role of angiogenesis during normal fetal development,51 bevacizumab should not be administered to pregnant women. Rituximab is a chimeric anti-CD20 IgG1 antibody that can cross the placenta and interact with fetal B cells. The few reports that have been published seem to indicate safe use of rituximab in pregnancy in all trimesters when given for both oncologic and nononcologic indications.16,18,21,23,26,31,52 Transient B-cell depletion in the neonate followed by full immunologic recovery and normal response to vaccinations has been reported in four cases.16,18,23,53 Rituximab is labeled pregnancy category C. Of the multitude of tyrosine kinase inhibitors available, the most published data regarding use during pregnancy pertains to imatinib, which is used to treat chronic myelogenous leukemia (CML). In a series of 180 women who were exposed to imatinib in pregnancy, with data available for 125, 12 infants were born with abnormalities, among which 3 had similar complex combinations of defects.28 On the basis of these data, imatinib cannot be recommended for use during pregnancy. Fewer data exist for dasatinib and nilotinib. Erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, is used in persons with metastatic lung and pancreatic cancer. Two case reports with no adverse outcomes have been published.54,55 No or little data are available on use of the following tyrosine kinase inhibitors during pregnancy: When administered during the first trimester, all-trans retinoic acid, which is used to treat acute promyelocytic leukemia, is associated with an 85% risk of teratogenicity, and when used with chemotherapy, it is associated with an increased rate of miscarriage.56 The use of all-trans retinoic acid alone or with chemotherapy in the second and third trimester has been shown to have generally favorable outcomes in the mother and fetus. Interferon alpha is believed to only minimally cross the placenta because of its high molecular weight. Forty cases of interferon used in pregnancy for a variety of hematologic disorders and eight cases in the first trimester showed no mutations. One case of fetal malformation was seen when used in conjunction with hydroxyurea.57 Minimal human data are available with respect to the following agents: • Topoisomerase-1 inhibitors such as topotecan (used to treat ovarian and small cell lung cancer) and irinotecan (used to treat colorectal cancer and non–small cell lung cancer) • Arsenic trioxide (used to treat acute promyelocytic leukemia) • Asparaginase (used to treat acute lymphocytic leukemia) • Bortezomib (a proteasome inhibitor used to treat multiple myeloma) • Bendamustine (an alkylating agent used to treat non-Hodgkin lymphomas) • 5-Azacitidine and decitabine (hypomethylating agents used to treat myelodysplastic syndromes) • Romidepsin and vorinostat (histone deacetylase inhibitors used in T-cell lymphomas) • Everolimus and temsirolimus (mTOR inhibitors used to treat renal cell cancer) Regarding the prevention of nausea and vomiting, consensus on the safe use of metoclopramide, 5-HT3 antagonists, NK1 antagonists, and corticosteroids during pregnancy have been established.58 For corticosteroids, methylprednisolone or hydrocortisone are extensively metabolized in the placenta, and thus relatively little crosses into the fetal side.59 Methylprednisolone and hydrocortisone are preferred over dexamethasone/betamethasone both for use as an antiemetic and for the prevention of anaphylaxis. Granulocyte colony-stimulating factor (G-CSF) is not teratogenic in rats, and no congenital malformations or toxicities attributable to G-CSF have been reported in humans.9 G-CSF is a category B drug and should not be withheld during pregnancy if there is significant potential benefit to the mother. Safe use in pregnancy has been documented,60 as well as in the prevention of recurrent miscarriage.61 Recombinant human erythropoietin (category C) does not cross the human placenta and does not appear to present a major risk to the fetus.13
Special Issues in Pregnancy
Introduction
Fetal Development and Physiology
Maternal Physiology: Relevance to Chemotherapy and Surgery
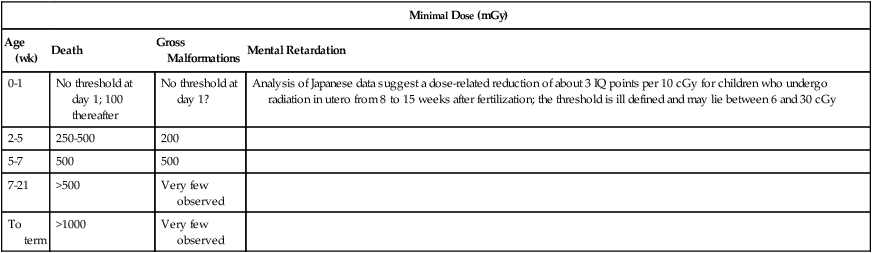
Diagnostic Radiology for Staging
Minimal Dose (mGy)
Age (wk)
Death
Gross Malformations
Mental Retardation
0-1
No threshold at day 1; 100 thereafter
No threshold at day 1?
Analysis of Japanese data suggest a dose-related reduction of about 3 IQ points per 10 cGy for children who undergo radiation in utero from 8 to 15 weeks after fertilization; the threshold is ill defined and may lie between 6 and 30 cGy
2-5
250-500
200
5-7
500
500
7-21
>500
Very few observed
To term
>1000
Very few observed
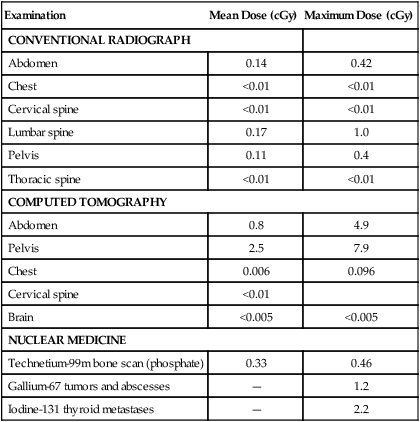
Examination
Mean Dose (cGy)
Maximum Dose (cGy)
CONVENTIONAL RADIOGRAPH
Abdomen
0.14
0.42
Chest
<0.01
<0.01
Cervical spine
<0.01
<0.01
Lumbar spine
0.17
1.0
Pelvis
0.11
0.4
Thoracic spine
<0.01
<0.01
COMPUTED TOMOGRAPHY
Abdomen
0.8
4.9
Pelvis
2.5
7.9
Chest
0.006
0.096
Cervical spine
<0.01
Brain
<0.005
<0.005
NUCLEAR MEDICINE
Technetium-99m bone scan (phosphate)
0.33
0.46
Gallium-67 tumors and abscesses
—
1.2
Iodine-131 thyroid metastases
—
2.2
Ultrasound, CT Scan, and Magnetic Resonance Imaging
Position Emission Tomography Scanning
Teratogenicity of Chemotherapy
Category
Description
A
Controlled studies in women do not show risk to the fetus during the first trimester; there is no evidence of risk in late trimesters, and the possibility of fetal harm is remote
B
Animal reproduction studies have not shown a fetal risk, but no controlled studies in pregnant women have been performed; or animal reproduction studies have shown an adverse effect (other than a decrease in fertility), but this effect has not been confirmed in controlled studies in women in the first trimester (no evidence exists of a risk in later trimesters)
C
Studies in animals have revealed adverse effects on the fetus (teratogenic, embryocidal, or both), and no controlled studies have been performed in women, or studies in animals and women are unavailable; drugs should be given only if potential benefit justifies the risk to the fetus
D
Positive evidence of human fetal risk exists, but the benefits from use in pregnant women may be acceptable despite the risk (if the drug is needed in a life-threatening situation for which other safer drugs are not available)
X
Studies in humans and animals have shown fetal malformations; there is evidence of fetal risk based on human experience or both; the risk of use in a pregnant woman clearly outweighs any potential benefit; this drug is contraindicated in women who are or may become pregnant
Specific Chemotherapy Drugs
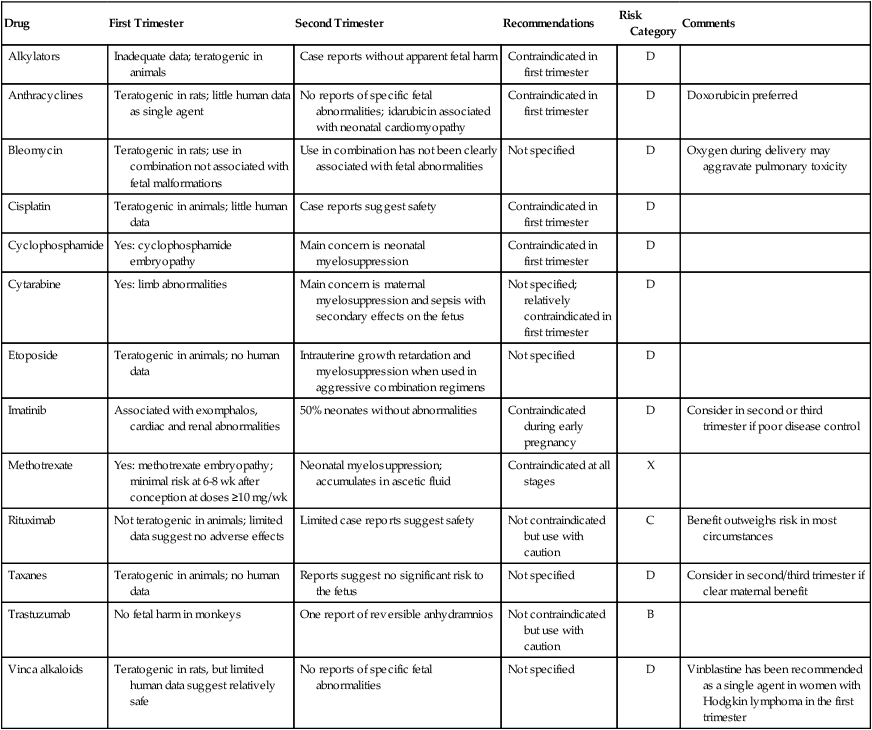
Drug
First Trimester
Second Trimester
Recommendations
Risk Category
Comments
Alkylators
Inadequate data; teratogenic in animals
Case reports without apparent fetal harm
Contraindicated in first trimester
D
Anthracyclines
Teratogenic in rats; little human data as single agent
No reports of specific fetal abnormalities; idarubicin associated with neonatal cardiomyopathy
Contraindicated in first trimester
D
Doxorubicin preferred
Bleomycin
Teratogenic in rats; use in combination not associated with fetal malformations
Use in combination has not been clearly associated with fetal abnormalities
Not specified
D
Oxygen during delivery may aggravate pulmonary toxicity
Cisplatin
Teratogenic in animals; little human data
Case reports suggest safety
Contraindicated in first trimester
D
Cyclophosphamide
Yes: cyclophosphamide embryopathy
Main concern is neonatal myelosuppression
Contraindicated in first trimester
D
Cytarabine
Yes: limb abnormalities
Main concern is maternal myelosuppression and sepsis with secondary effects on the fetus
Not specified; relatively contraindicated in first trimester
D
Etoposide
Teratogenic in animals; no human data
Intrauterine growth retardation and myelosuppression when used in aggressive combination regimens
Not specified
D
Imatinib
Associated with exomphalos, cardiac and renal abnormalities
50% neonates without abnormalities
Contraindicated during early pregnancy
D
Consider in second or third trimester if poor disease control
Methotrexate
Yes: methotrexate embryopathy; minimal risk at 6-8 wk after conception at doses ≥10 mg/wk
Neonatal myelosuppression; accumulates in ascetic fluid
Contraindicated at all stages
X
Rituximab
Not teratogenic in animals; limited data suggest no adverse effects
Limited case reports suggest safety
Not contraindicated but use with caution
C
Benefit outweighs risk in most circumstances
Taxanes
Teratogenic in animals; no human data
Reports suggest no significant risk to the fetus
Not specified
D
Consider in second/third trimester if clear maternal benefit
Trastuzumab
No fetal harm in monkeys
One report of reversible anhydramnios
Not contraindicated but use with caution
B
Vinca alkaloids
Teratogenic in rats, but limited human data suggest relatively safe
No reports of specific fetal abnormalities
Not specified
D
Vinblastine has been recommended as a single agent in women with Hodgkin lymphoma in the first trimester
Antimetabolites
Alkylating Agents
Platinum Derivatives
Taxanes
Vinca Alkaloids
Anthracyclines
Monoclonal Antibodies
Tyrosine Kinase Inhibitors
Other Agents
Supportive Care
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Special Issues in Pregnancy