Cardiopulmonary Physiology
Concerns regarding possible changes in cardiopulmonary physiology have been focused on maintenance of orthostatic function and physical work capacity. Orthostatic function is of concern during entry and landing. Maintenance of adequate physical work capacity is important for on-orbit activities as well as potential emergency egress during landing. Pulmonary changes
per se have not been very notable. Gravity affects the mechanical properties of the lung and chest wall, and changes have been reported in microgravity. Early studies in Skylab suggested that vital capacity (
VC) was reduced by approximately 10% during sustained spaceflight (
1). Reduced
VC may have resulted from the low environmental pressure of 258 mmHg or the slightly oxygen-enriched atmosphere (partial pressure of oxygen 170 mmHg). A 4% reduction in forced vital capacity (FVC) was also seen in 1 G under similar environmental conditions. Pulmonary
function was examined during shuttle research missions; peak expiratory flow rate, compared with preflight standing values, was reduced by 12.5% on flight day 2 (FD2) but returned to normal by FD9 (
3). FVC and forced expired volume in 1 second (FEV
1) were slightly reduced on FD2 but returned to normal by FD5 and were slightly increased by FD9. Analysis of the maximum expiratory flow-volume curve showed that microgravity caused no consistent change in the curve configuration when individual in-flight days were compared with preflight standing curves. The reduction in FVC and
FEV1 during the early phase of exposure is probably due to an increase in intrathoracic blood volume caused by the cephalad shift of body fluids. In summary, no physiologically significant changes in pulmonary function have been observed during spaceflight to date.
Obtaining valid data during flight activities is more complex than in the normal research laboratory environment. Changes in diet, sleep patterns, exercise, medications, and fluid intake before and during spaceflight missions are difficult to control. Safety restrictions may make many standard research protocols inadvisable. Data collections must occur without disruption of primary mission objectives. Hardware malfunctions during in-flight data collections affect the quantity and/or quality of results. Over the last three decades, symptoms of cardiovascular changes have ranged from postflight orthostatic tachycardia and decreased exercise capacity to serious cardiac rhythm disturbances. The most documented symptom of cardiovascular dysfunction, post-flight orthostatic intolerance, has affected a significant percentage of U.S. astronauts (25% for short duration missions and up to 80% for Shuttle-Mir astronauts). A basic parameter, heart rate, had previously been reported as increased, decreased, or unchanged during spaceflight. This important information deficit was corrected through systematic studies of Holter monitoring, blood pressure measurements, and related assessments of dysrhythmias, cardiac function, and orthostatic intolerance (
Figure 24-1).
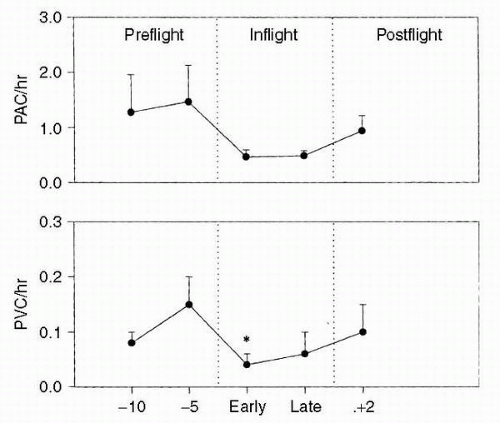
In a related descriptive study, 32 astronauts were evaluated with two-dimensional, directed M-mode echocardiography to determine the effects of spaceflight on cardiac volume, cardiac function, and cardiac mass. In a more operationally oriented study, 34 astronauts were instrumented with an automatic blood pressure/heart rate monitor that would permit data acquisition while the crewmembers wore their launch and entry suits (
LES). The following parameters were obtained: heart rate; systolic, diastolic, and mean arterial pressure; and pulse pressure. Several important findings resulted from these descriptive studies. First, it was shown that heart rate, diastolic pressure, and their variabilities were reduced during spaceflight (
4). Second, the diurnal variations of both heart rate and diastolic pressure were reduced during spaceflight. Third, monitoring records demonstrated that short-duration spaceflight did not increase dysrhythmias. Echocardiographic data showed some minor, statistically significant changes. Ejection fraction and velocity of circumferential fiber shortening did not change significantly, suggesting that spaceflight of this duration has no effect on myocardial contractility. Left ventricular wall thickness and myocardial mass index also showed no significant changes.
Standing upright for the first time after landing is associated with a significant decrease in systolic pressure. In several instances, the decrease was greater than 20 mmHg. This occurred in 22% of the subjects on landing day, but did not occur in any subjects before flight (
2). Therefore, this observed decrease in systolic pressure reflects the cardiovascular response of individuals adapted to shortduration microgravity. Many subjects used a pressurized anti-G suit during landing to support cardiovascular function. The recommended minimum inflation pressure for this protective garment was 26 mmHg or 0.5 lb per square inch guage (
psig). Most subjects inflated the suit to 52 mmHg; this counterpressure would largely offset the hydrostatic column effect of standing in a normal gravity environment. Diastolic pressure was more adequately maintained in those who inflated their anti-G suits. There was a 70% increase in heart rate upon standing compared with the increase seen before flight. The mean standing heart rate was 120 beats/min, but a maximum of 160 beats/min was observed in one stressed crewmember.
An important cardiovascular study focused on an integrated assessment of orthostatic function (
2). There were 29 participants, and 8 could not complete a 10-minute stand test on landing day because they became presyncopal (
2). These subjects displayed arterial pressure and heart rate responses to standing that were similar to those seen in adrenergic failure. On landing day, their standing norepinephrine levels were significantly lower than the norepinephrine levels of the astronauts who did not become presyncopal. Plasma volumes (PVs) were not significantly different between the two groups. The group that became presyncopal on landing day had lower preflight supine and standing diastolic
pressures and lower peripheral vascular resistance than the nonpresyncopal group. These data taken as a whole provide convincing evidence that the precipitating factor for orthostatic intolerance after spaceflight was a hypoadrenergic response to orthostatic stress. The parallel insufficient levels of plasma norepinephrine, diastolic pressure, and peripheral vascular resistance strongly support this. Removal of all hydrostatic gradients when entering microgravity of spaceflight produces a large headward fluid shift; this has been commonly observed and is reflected in measurements of decreased leg volume and observations of an edematous face. This fluid shift is believed to be the primary stimulus for many of the physiologic effects of spaceflight, including a reduced PV and, ultimately, orthostatic intolerance on return to Earth’s gravity. It has been hypothesized that central venous pressure (
CVP) would increase due to the headward fluid shift encountered upon entering microgravity. Direct measurements were made by catheter, and the results were somewhat surprising:
CVP was 8.4 cm H
2O seated before flight; 15 cm H
2O in the supine legs-elevated posture before launch in the shuttle; and 2.5 cm H
2O after 10 minutes in space (
5). A corollary measurement of left ventricular end-diastolic dimension measured by echocardiography showed an increase from a mean of 4.6 cm supine preflight to 5.0 cm within 48 hours in space.
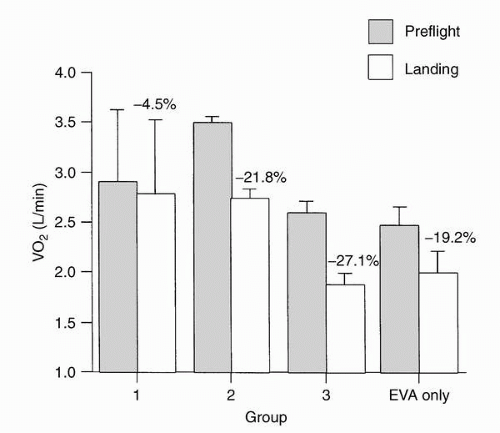
Other investigators have evaluated the cardiovascular response to submaximal exercise in spaceflight (
6). Cardiac output, heart rate, blood pressure, and oxygen consumption were measured repeatedly both at rest and at exercise levels approximating 30% and 60% preflight maximum. Cardiac output at rest in-flight was an average 1.5 L/min greater than erect preflight, but not different from supine preflight values. Inflight resting heart rate was an average 15 beats/min lower than the erect control value and 5 beats/min lower than the supine control value. Inflight stroke volume was elevated relative to both erect and supine control values. Inflight mean arterial blood pressure was 6
mm Hg less than erect control values, but not different from supine control values. In spaceflight, the increase in cardiac output with oxygen consumption was due entirely to an increase in heart rate. Stroke volume was not a linear function of oxygen consumption. Total peripheral resistance in-flight at rest was lower than that erect preflight but not different from that supine. One of the mechanisms responsible may have been hydrostatic unloading of dependent veins. The subjects were able to exercise in microgravity until they achieved their control maximal heart rate and maximal oxygen consumption. Mean cardiac power, expressed as the product of cardiac output and mean arterial pressure (triple product) does not appear to be a limiting factor in the increase of cardiac output with increasing levels of exercise in-flight. The maximal stroke volume seen in-flight occurred at rest; stroke volume actually declined with increasing oxygen consumption (
6).
Cardiovascular countermeasure studies were conducted to determine if early inflation of the standard five-bladder anti-G suit before centrifuge simulation of shuttle landing would provide better protection against orthostasis than the standard symptomatic inflation regimen (
2). Preinflation to at least 0.5
psig protected eye-level blood pressures better and resulted in lower maximum heart rates during these simulations. Although preinflation subjects exhibited a greater decrease in systolic pressure during centrifugation (−51
mm Hg vs. −36
mm Hg), they completed the runs with higher absolute systolic pressures. These findings resulted in a flight rule that now requires preinflation of anti-G suits before reentry.
The National Aeronautics and Space Administration (
NASA) evaluated a liquid cooling garment (
LCG) as a countermeasure to the thermal load imposed by the
LES. This thermal load was believed to be largely responsible for the increased incidence of orthostatic intolerance (˜25% pre-Challenger and 52% post-Challenger) noted following their introduction as standard garments following the Challenger accident in January 1986. The metabolic heat produced by an average astronaut is approximately 100 W/hr. Before use of the
LCG, body heat could be dissipated only by circulating cabin air across the chest within the
LES garment. This provided modest benefit in a cool cabin and no benefit after landing when cabin air temperatures often reached 27°C to 32°C. The
LCG uses a thermoelectric cooler to chill water before circulating it through a full-torso, tube-filled garment. The
LCG is presently worn under the new advanced crew escape suit (
ACES) for launch and landing; it has proved extremely effective both for general comfort and orthostatic protection. Weight loss postflight averaged 0.7 kg less for crewmembers who wore the
LCG. This difference probably represented decreased water loss due to lower sweat rates and respiratory loss during entry and landing activities. The frequency of orthostatic symptoms has decreased to pre-Challenger levels.
Alternative fluid loads were evaluated because the standard 8 g of sodium chloride diluted to be approximately isotonic in a liter of water evoked nausea and emesis in many subjects, which in turn led to decreased compliance with the flight rule requiring fluid loading before entry. Ground-based studies determined that isotonic fluids were essential. Either isotonic consommé or Astroade (potassium citrate instead of sodium chloride) was equally beneficial. Fluid loads containing natural sugars were not as effective because they induced diuresis. Hypertonic solutions often caused diarrhea. Therefore, the standard fluid load is now an isotonic fluid, 15 mL/kg preflight body weight, taken 2 hours before landing.
Experience gained during shuttle operations raised significant concerns regarding orthostatic tolerance for crewmembers returning from 4- to 6-month missions on Mir.
Soyuz vehicles return their cosmonauts in the supine position with the G-load from chest to back (positive G
x). If a normally seated astronaut or cosmonaut were to become orthostatically intolerant during entry and shuttle landing, there could be an approximate 15-minute period when no assistance would be possible. Therefore, a decision was made to use a conservative approach and return long-duration crew in the supine posture until sufficient experience had
been gained to determine that this would not be necessary.
NASA designed, manufactured, and provided the recumbent seating system (
RSS) for use on Shuttle/Mir missions. Its use has been extended to the
ISS for returning long-duration crews (missions longer than 30 days). This system accommodates up to three crewmembers in the shuttle mid-deck. It was first used for the
STS-71/Mir 18 mission to return one astronaut and two cosmonauts from their 115-day mission. Each subject also wore an anti-G suit that was inflated to the nominal pressure of 75 mmHg (1.5
psi). Heart rates were approximately 25 beats/min lower than those for seated shuttle crewmembers on previous missions. Upon first standing, the crew from Mir demonstrated comparable heart rates to those from the earlier, significantly shorter shuttle missions. Systolic and diastolic blood pressures were slightly lower in the Mir crew relative to shuttle crews. These two findings indicate the protective benefit of the countermeasure. The fact that upon first standing the two groups displayed similar cardiovascular responses is encouraging; it indicates that the general cardiovascular status of the returning Mir crew was comparable with that of shuttle crewmembers.
Exercise physiologywas studied because it was recognized that both physical and psychological benefits were received from in-flight exercise sessions. These investigations resulted in establishment of a flight rule requiring exercise on missions greater than 10 days in duration. A key objective was to determine the optimal combination of a crewmember’s fitness before flight and in-flight countermeasures that would result in minimal performance decrements. Conducting well-controlled investigations proved extremely difficult due to multiple conflicting priorities during each mission. In general, moderate to more intense levels of cycle exercise resulted in improved submaximal exercise responses after flight. This response required exercising more than three times per week for greater than 20 minutes per session at intensities of 60% to 80% preflight maximum work loads (
2) (
Figure 24-2).
One suggested countermeasure, based on ground-based bed rest studies, required a single bout of maximal, cycle exercise 24 hours before landing. This was postulated to improve post-flight aerobic exercise response and orthostatic tolerance. Results from the initial subjects did not support the hypothesis, and further trials were canceled (
2). Significant additional effort is still required to define an optimal exercise program that gives consideration to aerobic versus resistive; upper body versus lower body; eccentric (force while lengthening) versus concentric (force while shortening); high-intensity interval versus low-intensity continuous protocols; and high impact versus low impact. This information is required to determine an appropriate balance of crew risk and discomfort versus the expected physiologic benefit of the exercise.
Neurovestibular Physiology
A significant concern for operational spaceflight is the occurrence of space motion sickness (
SMS). Attempts in the Russian space program to prevent
SMS by selecting individuals with a high tolerance to vestibular simulation have not been successful. Applicants to the astronaut corps are not screened for motion sickness resistance. Moreover, among crewmembers who have completed preflight Coriolis testing, no correlation has been found between test tolerance and susceptibility to
SMS.
SMS occurs typically during the initial 3 days of spaceflight. No single drug or combination of drugs has been proved to protect all people. Promethazine, the most effective of the antihistamines, has proved very effective when taken by intramuscular injection or as a suppository. An injection of 25 to 50
mg of promethazine is now the recommended treatment for moderate to severe
SMS in the U.S. space program (
7,
8). In summary, results obtained in both the U.S. and Russian space programs indicate that most spacecrews will experience some symptoms of
SMS (>70%), and that these symptoms are brought on or made worse by head movements. These symptoms may be debilitating, and their probability of occurrence has led to operational rules that preclude
EVA during the first 3 mission days. All but two cosmonauts following long flights and 27% after short flights showed symptoms on the first and second days after landing, and sometimes on the next few days as well, that could be classified as clinical vestibular dysfunctions. These symptoms consisted of illusions (e.g., dizziness, illusory movement of self or surroundings), motor reactions (e.g., pointing errors), and vestibular reactions (e.g., nystagmus of central or sometimes peripheral nature), which varied in severity. On the first day after landing, all cosmonauts complained of instability upon standing and of “swaying” from side to side while walking (
9).
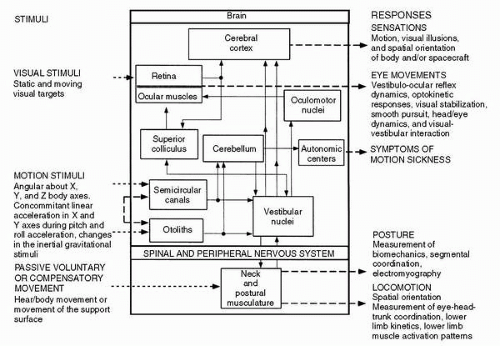
Flight surgeons (FSs) frequently observed disequilibrium in crewmembers during the first few hours after spaceflight. These observations were in large part attributed to functional changes in the neurovestibular system. Four primary research goals to investigate the neurovestibular system were (a) to establish a normative database of vestibular and associated sensory changes in response to spaceflight, (b) to
determine the underlying etiology of neurovestibular and sensory-motor changes associated with exposure to microgravity and the subsequent return to Earth, (c) to provide immediate feedback to spaceflight crews regarding potential countermeasures that could improve performance and safety during and after fight, and (d) to design appropriate countermeasures that could be implemented for future missions. The neural processes that mediate human spatial orientation and the sensory rearrangement encountered during orbital flight are optimally studied through second- and third-order responses. The following parameters were measured during separate studies: (a) eye movements during acquisition of either static or moving visual targets; (b) postural and locomotor responses provoked by unexpected movement of the support surface, changes in the interaction of visual, proprioceptive, and vestibular information, changes in the major postural muscles through descending pathways, or changes in locomotor pathways; and (c) verbal reports of perceived self-orientation and self-motion that enhance and complement conclusions drawn from the analysis of oculomotor, postural, and locomotor responses. Spatial orientation in this context is defined as situational awareness, where a crewmember’s perception of attitude, position, or motion of the spacecraft or other objects in three-dimensional space, including orientation of one’s own body, is different from actual physical events. The interaction of stimuli and responses is illustrated in
Figure 24-3 (
2).
Perception of spatial orientation is determined by integrating information from several sensory modalities. This involves higher levels of processing within the central nervous system to control eye movements, stabilize locomotion, and maintain posture. Operational problems occur when reflex responses to perceived spatial orientation lead to inappropriate compensatory actions. Future application of effective countermeasures depends, in large part, on the interpretation of results from appropriate neuroscience investigations. A number of experimental paradigms, classified as voluntary head movements (
VHMs), included the performance of (a) target acquisition, (b) gaze stabilization, (c) pursuit tracking, and (d) sinusoidal head oscillations. Target acquisition protocols used a cruciform tangent system where targets were permanently fixed at predictable angular distances in both the horizontal and vertical planes. To facilitate differentiation, each target was color coded (e.g., ±20 degrees green; ±30 degrees red, etc.) corresponding with the degree of angular offset from center. The subject was required to use a time optimal strategy for all target acquisition tasks, and to look from the central fixation point to a specified target indicated by the operator (e.g., right red, left green, up blue, etc.) as quickly and accurately as possible using both head and eye movement to acquire the target. During flight, measurements were obtained using a cruciform target display that attached to the shuttle mid-deck lockers. In all cases, surface electrodes on the face enabled quantifying eye movements that were obtained with both horizontal and vertical electrooculography. Head movements were detected with a triaxial rate sensor system mounted on goggles that
were fixed firmly to the head. Both head movements (using a head-mounted laser) and eye movements were calibrated.
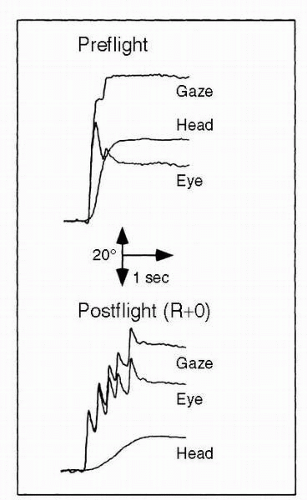
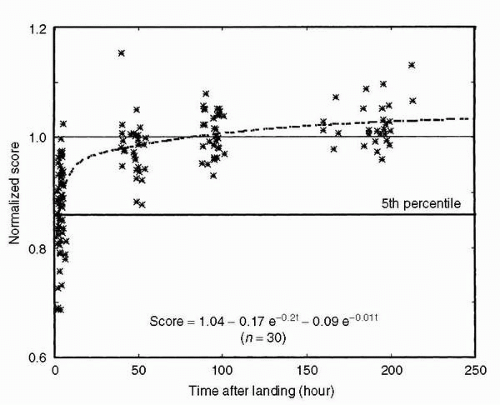
Pursuit tracking, (i.e., visually moving from a central focal point to illuminated targets) was performed before flight and after flight, using two separate protocols: (a) smooth pursuit by the eyes only, and (b) pursuit tracking with the head and eyes together. In addition, a modification of these protocols used predictable, sinusoidal stimuli and unpredictable stimuli with randomly directed velocity steps. The objective was to study smooth pursuit eye movement and to evaluate its interaction with the vestibuloocular reflex. The sinusoidal pursuit tracking tasks were performed at moderate (0.33
Hz) and high (1.4
Hz) frequencies to investigate the relative contributions of eye and head movement in maintaining gaze. Significant difficulties were observed postflight, including multiple saccades (
2) (
Figure 24-4).
Consequently, the time required to clearly focus an image of the target on the retina increased by as much as 1 to 1.5 seconds relative to preflight times. This important finding led to discussions with shuttle commanders suggesting that they limit in particular their vertical head movements to monitor instruments during approach and landing.
Spatial orientation was tested in several ways. First, it was of interest to determine whether in space the brain is able to detect objects moving at a constant velocity as opposed to those moving at constant linear acceleration. It was found that subjects are able to successfully change their strategy catching falling objects in space. They apparently predict the velocity of the ball and also its momentum, and make an upward hand movement to catch the ball. Hand-eye coordination was tested in reaching, pointing, and grasping tasks. Amplitude, reaction time, duration, and velocity were studied while reaching for an X-Y position without seeing the hand. The amplitude of the movements was the same on earth as in-flight, but there was a slight increase in reaction time and duration, and a decrease in velocity. Two protocols investigating postural stability were performed by 40 crewmembers before, during, and after shuttle missions of varying duration. These tests used a clinical Neurocom posture platform to challenge the subject’s ability to maintain balance by six different sequential tests. The first of these protocols focused primarily on reactive responses by quantifying the reflex (open-loop) response to sudden stability-threatening perturbations of base support. The second protocol focused on sensory integration by quantifying the postural sway during quiet upright stance with normal, reduced, and altered sensory feedback. After flight, tests began on landing day, as soon after the shuttle wheels stopped as possible, and were scheduled on an approximately logarithmic time scale during the subsequent 8 days (
Figure 24-5).
The effect of spaceflight on neural control of posture was inferred from differences between preflight and postflight performance in all subjects. The effect of mission duration was inferred from statistical comparison between the performance of subjects on short-, medium-, and long-duration missions. The effect of previous spaceflight experience was inferred from statistical comparison between the performance of the rookie and veteran fliers. Astronauts with previous flight experience demonstrated better postural stability that suggested retained neurosensory learning. Centrifugation in microgravity produces linear accelerations that mimic the pull of gravity. On Earth, subjects feel tilted to the side during centrifugation that produces linear acceleration along an interaural axis, and tilted back during centrifugation when lying on their backs. This is attributable to the sum of the upward gravitational linear acceleration with the centripetal acceleration from centrifugation. On landing, there was enhanced perception of tilt. Oculomotor measures of otolith-ocular reflexes, such as ocular counterrolling, were similar before, during, and after flight. Spatial orientation to the vestibuloocular reflex in response to centripetal linear acceleration appeared to be maintained throughout the flight. These findings demonstrate that there is a strongly developed sense of orientation to gravity that persists in space (
10).
Bone and Muscle Physiology
Bone and muscle respond to microgravity within the first few days of a space mission. In space, the mechanical load, or amount of weight that bones must support, is reduced almost to zero. At the same time, many bones that aid in movement are no longer used as intensely as they are on Earth. These characteristics of life in microgravity evoke two of the biggest concerns regarding the human body during spaceflight: disuse osteoporosis and muscle atrophy. Bones are relieved of mechanical loads normally experienced on Earth, and calcium normally stored in bones (which gives bones strength) is broken down and released into the bloodstream. This decrease in density, or bone mass, is called
disuse osteoporosis. The exact mechanism by which the body divests itself of bone mass is still unknown. Current theories point to the fact that in space, old bone is absorbed much faster than new bone is laid down. The changes in bone density begin within the first few days in space. The most severe loss occurs between the second and fifth months in space, although the process appears to continue throughout the entire stay in microgravity. Bone demineralization is tracked during a mission by monitoring electrolytes; calcium in the urine serves as one marker of bone loss. Baseline bone mineral density, bone loss, and recovery are monitored preand postflight through imaging techniques such as dual-energy x-ray absorptiometry (
DEXA). These measurements have documented losses in the lower spine and hip, posterior elements of the vertebrae, femoral neck, and tibia. Recovery of bone mass may take up to 2 years despite the fact that excess calcium no longer appears in urine soon after return to Earth. The risk of injury to long-duration flight crews is compounded by coordination and balance difficulties typically experienced upon return to Earth. The potential for falling and fracturing a bone is much higher than normal following flight, especially in those with a lower bone mass and reduced fracture threshold.
As bone mass is lost in microgravity, muscle undergoes a similar process. Numerous studies throughout the history of spaceflight have documented changes to muscle mass and architecture. In essence, the muscles used in standing, walking, and posture maintenance on Earth are relatively unused in space. These muscles begin to atrophy, becoming smaller and weaker. As a result, astronauts may lose strength and size in their muscles, ultimately extending the time for recovery of bone mass. Again, the exact mechanism behind this change remains elusive. Bone loss in spaceflight is much more accelerated than bone loss on Earth. Gender differences in spaceflight are also less pronounced than on Earth; in space, both men and women lose bone mineral at approximately the same rate. The human musculoskeletal system is a complex, multicomponent array of effector organs (including muscles), connectors (such as tendons and ligaments), and structural components (bones) responsible for the support and movement of the human body. The skeletal system provides the mechanical support to which muscles attach for movement, protects the internal organs, houses the bone marrow, and stores calcium, phosphorous, and other ions. The skeleton allows us to remain upright and move in the presence of gravity. In the presence of gravity, our bipedal stance and gait dictate that certain bone and muscle groups are essential to posture and movement. The muscles of the legs and trunk are responsible for producing the forces required for activities such as walking, running, and maintenance of an upright posture in a terrestrial setting. In contrast, arm bones and muscles are responsible for providing the forces required for activities associated with upper body balance and manual dexterity. Consequently, the functioning of bone and muscle are closely linked. It is therefore not surprising that environmental conditions affecting bone impact muscle as well. Bone is a highly vascular, constantly changing, specialized type of mineralized connective tissue consisting of two types of bone cells. Osteoblasts, cells that lay down new bone material, are responsible for bone formation, whereas the osteoclasts reabsorb old bone material. Formation and destruction of bone is an ongoing process throughout life; we typically replace approximately 20% of our bone each year. The loss of bone in space appears to be related to an adaptive process where the body senses it does not need as much bone as when it was on the ground. The hormonal regulation of calcium and bone metabolism reflects this change. At the cellular level, the decreased calcium absorption is related to decreases in circulating vitamin D. Researchers have also observed that decreases in certain hormonal levels (e.g., growth-stimulating hormone that affects the parathyroid gland) correlate with decreased bone formation. Generally accepted theory holds that our endocrine system responds to microgravity by disrupting the balance between bone resorption and bone construction, such that resorption
exceeds construction. In addition, it is assumed that genetic factors also play a role in determining the extent to which bone is lost during weightlessness. Scientists have extensively evaluated the minerals that provide bones with their strength. Calcium is the primary mineral required for strong bones, so researchers have studied the amount of calcium in the body and its movement throughout the body. The excretion of calcium and phosphorous during spaceflight is increased. The rate of calcium loss from bone increases during flight. The rate of bone calcium loss is approximately 250
mg/d, which calculates to 1% to 2% mass loss per month in the lower extremities. Recovery after spaceflight is approximately 100
mg/d; therefore, the recovery of lost bone mass will take approximately 2.5 times as long as the mission duration.
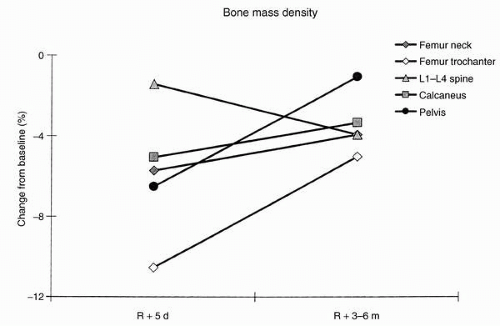
NASA researchers scanned the bones of 19 Russian cosmonauts who flew between 126 and 312 days on the Mir space station. Bone loss in the spine (>1% per month) exceeded that seen in bed rest subjects. Decreases in bending and torsional strength, averaging approximately 8% in the femoral neck and approximately 4.5% in the femoral shaft, also were measured. Together, these data suggest that countermeasures for maintenance of bone integrity were inadequate. Changes in bone mineral density after astronauts participated in 4- to 6-month flights on the Mir space station are shown in
Figure 24-6.
Density of the skeleton was measured within the first week following flight. Mean loss was −11% in the trochanter area of the femur, −6% in the neck of the femur, −7% in the calcaneus of the heel, −7% in the pelvis, and −1% in the lumbar spine. It was documented that lumbar spine losses continued postflight for up to 6 months as other regions recovered. Final recovery to preflight density occurred for five of seven subjects. Aerobic and to some degree resistive exercises have been the primary countermeasures against bone loss throughout the history of spaceflight, but they have been only partially effective. Although the Skylab-3 and Skylab-4 crews as well as the Mir crews performed extensive aerobic exercise during flight, they showed substantial mineral losses (
1). This has cast doubt on the effectiveness of the exercise program. Additional, intensive resistive exercise programs are under evaluation. New countermeasures will likely include resistive exercise directed at specific affected areas, and possibly
AG. Resistive exercise is difficult to accomplish in space, and
AG requires major spacecraft hardware considerations. Potential pharmacologic countermeasures are based on a class of drugs referred to as
bisphosphonates; these drugs block the action of osteoclasts and are currently under investigation in bed rest studies (
Figure 24-7).
The link between bone loss and muscle atrophy is widely accepted. The absence of mechanical strain on the lower limbs during spaceflight leads to a rapid decline in muscle mass. This decrease in muscle mass and strength appears to be a contributing factor to bone loss in space. The exact mechanism of these muscular effects on bone is unknown. There are indications of alterations in molecules used by the body to transmit information about a change in mechanical strain. For example, studies have demonstrated that levels of interleukins and insulin-like growth factor I decrease in microgravity and therefore enhance bone resorption. Microgravity also promotes enhanced absorption of calcium directly from bone, which further affects hormonal levels. It is possible that specific cells in the bone marrow may promote the formation of bone-resorbing osteoclasts. In addition, bone loss is thought to be progressive; the amount of bone loss in weight-bearing bones seems proportional to the length of flight. Unfortunately, bone loss occurs despite
available countermeasures. It is projected that the loss will stop after some time, but we have yet to reach that point in the flight studies to date.
Mammalian skeletal muscle has a standard architecture developed around the contractile protein elements responsible for force production, namely
actin and
myosin. It is the actin-myosin “molecular engine” that is responsible for producing the force developed during muscle contraction. The actual process of movement is accomplished through muscle contraction. Contraction is the result of a complex chemical reaction. When movement is needed, the brain releases a neurotransmitter that communicates an electrical impulse to the muscle fiber. Thousands of fibers contract to produce movement in the muscle. This process—from the time that the signal leaves the brain to the time that the muscle contracts—takes only minutes. Movement may also be the consequence of a reflex action. In this case, the brain is not part of the signal pathway; the spinal cord is the natural connection between a reflex action and movement of the muscle. All muscles are slightly contracted during consciousness; this is referred to as
tonus. Tonus contributes to the upright posture and keeps the muscles stimulated and healthy. Nonstimulation leads to muscle atrophy. Slowtwitch fibers are those normally associated with activities requiring endurance, such as long distance running, walking, or maintenance of posture. Fast-twitch fibers are those associated with muscle normally used for rapid movement such as sprinting or power lifting. One of the most important aspects of muscle physiology with regard to environmental challenges is the level and the type of mechanical load to which a particular muscle is exposed. Muscles adapt based on their loading histories. To better understand changes in muscle, researchers developed a breakthrough methodology to directly measure proteins involved in muscle synthesis. Direct measurement of protein synthesis rates in astronauts suggests that the normal balance maintained between protein synthesis and degradation is disturbed during spaceflight. Protein degradation rates must be significantly increased during spaceflight in order to account for the rapid loss of the muscle mass. Pre- and postflight imaging has shown dramatic reductions in skeletal muscle volume in a large number of muscle groups, including the soleus/gastrocnemius (calf muscle), hamstrings, and quadriceps (thigh) muscles after both short- (8 days) and long-duration (115 days) spaceflight. Reductions in both lean body mass and muscle volume are paralleled by a concomitant decrease in the cross-sectional area of the individual muscle fibers. Limited data, obtained from muscle biopsy from both American astronauts and Russian cosmonauts, show a reduction in muscle fiber with associated loss of contractile protein elements, actin and myosin. In addition, the loss of muscle mass is most prevalent in the antigravity muscles and is associated with a loss of muscle strength and endurance (
2). There is no doubt that the removal of mechanical load from the musculoskeletal
system is the most important initiating factor in skeletal muscle atrophy. Changes in muscle morphology were studied by pre- and postflight biopsy of the vastus lateralis muscle in the thigh. Significant changes were evident after 6- to 9-day shuttle missions, including a 15% reduction in the cross-sectional area of type 1 and a 22% reduction in cross-sectional area of type 2 muscle fibers. Decreased capillary density and increased ratio of glycolytic/oxidative enzymes resulted in muscle becoming relatively more anaerobic. Muscle function was measured by a Lido dynamometer. Large decrements in trunk flexor and extensor strength, both concentric and eccentric, and significant losses in concentric quadriceps extension were seen. Muscle strength typically recovered within 7 to 10 days, with the exception of concentric back extension following short-duration missions (
2).
Hematology
Astronauts consistently return from space with a decreased red blood cell mass (
RBCM) (
11). This has been observed during Apollo missions with a 258 mmHg (5 psia) oxygen atmospheric pressure, during Skylab with a normal sea-level oxygen partial pressure but lower total pressure environment (BP = 258 mmHg), and finally in shuttle missions where the atmospheric composition and pressure were sea-level equivalent. During detailed studies on Spacelab missions, the observed decrease in
RBCM was attributed to fewer new red blood cells (RBCs) being released from the bone marrow (
11). The hematocrit, reflecting changes in the
RBC count and mean corpuscular volume (
MCV), did not increase significantly during flight. Six days after flight,
RBC count, hemoglobin concentration, and hematocrit were all below preflight mean values. The mechanisms of action are thought to occur as follows. PV decreases, causing an increase in hemoglobin concentration that affects a decrease in erythropoietin or other growth factors or cytokines. The
RBCM decreases by destruction of recently formed RBCs to a level appropriate for the microgravity environment. This represents normal adaptation to microgravity. On return to Earth, there is acute hypovolemia as vascular space dependent on gravity is refilled, an increase in PV, a decrease in hemoglobin concentration (representing “anemia”), and an increase in serum erythropoietin. Because erythropoietin is either decreased or normal in-flight, it supports the thesis that decreased
RBCM is a normal adaptation to the microgravity environment. Changes after return to Earth, that is, orthostatic hypotension, rapid increase in PV, and increase in serum erythropoietin indicate that the optimal values for both plasma and RBCs are greater on Earth than in space. Normal
RBC survival was documented by use of circulating chromium-tagged RBCs. Typically 1% of RBCs is replaced daily. The increase in chromium-specific activity and decrease in
RBCM probably occurred as a consequence of not replacing cells that were normally destroyed.
Detailed studies of blood volume were not accomplished for the astronauts who flew 4- to 6-month missions on the Russian Mir station. However, interesting mean changes were measured pre- to postflight that were generally consistent with prior observations for other programs. Hemoglobin was decreased approximately 10% postflight. Hematocrit was decreased 4%, and the
RBC count was down approximately 6%. There was no apparent change in
MCV.
Nutrition
Skylab was a prototype space station flown in the early 1970s. There were three missions, Skylab-2, -3, and -4, with three astronauts each and lasting 28, 59, and 84 days, respectively. For each of the three Skylab missions, a metabolic balance study was performed beginning at least 21 days preflight and continuing until 17 days after return for the nine astronauts. Variables monitored included diet, fluid, and electrolyte balance, various hormones, and nitrogen balance. The Skylab investigators found that the protein loss was greatest during the first month, but continued for the duration of the mission (
1).
These Skylab data for the first 12 days were compared with studies conducted on 1- to 2-week Shuttle missions (
12). There was no statistically significant difference in the preflight dietary intake or estimated nitrogen balance between the two groups, although there may have been a trend for nitrogen balance to be less for the shuttle astronauts. Although energy intake was higher on Skylab, the nitrogen loss was greater. Over the entire duration of the Skylab missions, urinary nitrogen excretion was increased by 3.1 g/d above the preflight control period. With the shuttle, the decreased nitrogen balance paralleled the decreased protein intake (
12). Therefore, it is clear that energy intake and nitrogen balance were different between shuttle and Skylab, and when the estimated nitrogen balance is used as the criterion for comparison, shuttle crews fared better. Skylab astronauts individually developed a prescribed daily exercise regimen, which increased in intensity from Skylab-2 through Skylab-4. This daily exercise regimen was not required or prescribed. Each crewmember selected his own mix of equipment utilization and specific protocols with advice from ground monitors. For Skylab-2, only a cycle ergometer was available; for Skylab-3, an apparatus referred to as a
mini-gym that provided some resistive exercise was added. A simulated treadmill was added for Skylab-4. This device was a Tefloncoated metal plate that the subject slid his stocking-covered feet across while maintaining position with a bungee cord restraint harness designed for the ergometer.
It was uncertain how the combined effects of limited physical activity and perhaps increased stress would affect nutritional requirements. Earlier spaceflight studies indicated changes in protein turnover that were consistent with a stress reaction during shuttle flights. Estimated water turnover (important inflight due to concerns about the potential for renal stone formation) was a by-product of the nutritional studies. Various experts have recommended inflight water intake of at least 2,500 mL/d. Rehydrated food and fruit drinks provide approximately 80% of this fluid intake.
Energy expenditure requirements were determined for 13 male astronauts 36 to 51 years of age during spaceflights 8 to 14 days in duration (
2). Methods used were developed from the doubly labeled water (
DLW) technique modified to account for baseline isotopic differences associated with the shuttle potable water system. The analytic uncertainty of the
DLW method performed in ground-based laboratories is ±4.5% actual average metabolic rate. The slightly higher isotopic doses used for these studies reduced the uncertainty to approximately ±3.5%. Baseline metabolic studies were accomplished approximately 2 months before flight, whereas flight studies typically began on the third flight day to avoid confounding effects associated with
SMS. The energy requirements associated with physical activity in microgravity were largely unknown, and the relatively close confines of the spacecraft tend to limit the extent of physical activity. Ambient temperature and relative humidity are held relatively constant within the shuttle at 21°C to 24°C and 20% to 30%, respectively. During flight, energy intake (8.8 ± 2.3 MJ/day) was less than flight total energy expenditure (11.7 ± 1.9 MJ/day;
p <0.005). Body weight was less at landing than at 2 days before launch (76.5 ± 6 kg compared with 78 ± 6 kg, respectively;
p <0.05). No statistically significant differences were found between ground-based and inflight energy expenditures.
The loss of bodyweight has been a consistent findingwith spaceflight. Some of the weight loss is due to the loss of fluid, but there is also the loss of lean body mass and some loss of strength, particularly in the postural muscles. Astronauts are in negative nitrogen balance during spaceflight. The protein loss is of concern because it could limit the duration of human spaceflight. The principal approach to reducing the inflight muscle loss is to follow an aggressive, time-consuming exercise program inflight. Russian crews typically exercise for at least 2 hr/d. The costs of such a program are considerable in terms of the extra food, the capability for disposal of the metabolic waste products, and the time spent exercising. Although it is generally believed that exercise can attenuate the protein loss, there have been no specific inflight tests with appropriate controls to determine efficacy of current exercise regimens.
Any crewmember who is in negative energy balance (i.e., whose energy expenditure exceeds energy intake) will lose lean body mass regardless of the type, frequency, or intensity of exercise regimens or protein consumption. Results from Skylab suggest that crewmembers can and do lose weight during flight despite the consumption of adequate calories (energy) and protein. The weight lost as a result of microgravity exposure generally consists of body fluids and electrolytes, RBCs, and muscle or lean tissue. A significant portion of in-flight weight loss, even during relatively brief missions (up to 10 days) is thought to be due to loss of lean body mass. One important cause of loss of lean body mass is being in negative energy balance. Loss of lean body mass reduces muscle work capacity and promotes loss of electrolytes, especially potassium, which affects muscle and cardiovascular function (
12).
Interpretation of body weight changes during spaceflight was confounded by the fluid-loading countermeasure, although typically most fluid load was lost through a combination of diuresis and perspiration. Total weight loss, recorded at landing, reflects a combination of tissue and water loss. Furthermore, it has previously been shown that relative proportions of energy sources shifted during flight, with the carbohydrate component increasing, protein remaining stable, and fat declining. The poorer nitrogen retention for Skylab crews, despite greater food intake relative to the shuttle crews, suggests inadequate intake relative to added energy costs of the overall exercise. During spaceflight, as on the ground, inadequate energy intake coupled with exercise will exacerbate the loss of body protein. Energy costs of normal living in space are probably lower than on the ground for comparable activity. The difference between exercising and “normal living” in space is that the exercise has to be done in a prescribed way, whereas with normal living the subject is free to use multiple modes. An inflight exercise program may be counterproductive for attenuating the spaceflight-induced
protein loss unless the associated increased energy needs can be met.
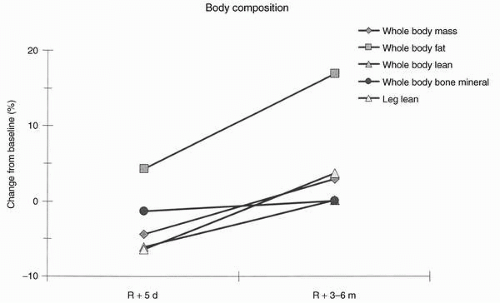
Figure 24-8 summarizes mean changes in body composition as measured postflight in seven Shuttle-Mir astronauts.
Renal and Gastrointestinal Physiology
Exposure to the microgravity environment of space produces a number of physiologic changes of both metabolic and environmental origin that increase the potential for renal stone formation. Metabolic, environmental, and physicochemical factors that influence the risk for developing renal stones were examined in 24-hour urine samples from astronauts 10 days before launch and on landing day, to provide immediate assessment of these factors (
2). Decreased fluid intake and vomiting associated with
SMS in some people during early phases of spaceflight probably contribute to decreases in urine volume, thereby increasing the risk of stone formation. Urinary calcium levels increase during flight, reflecting the overall negative calcium balance. Several of the physiologic changes that take place during human exposure to microgravity are thought to affect the factors that contribute to the risk of renal stone formation, especially changes in urine volume or urinary calcium, phosphate, potassium, and sodium excretion. For example, urinary calcium concentrations begin to increase within 24 hours of exposure to microgravity; urinary phosphate levels also tend to be higher during flight than before. Both of these imbalances probably reflect the bone demineralization process, and both increase the potential for urinary saturation of calcium oxalate and calcium phosphate. Additional factors that could aggravate stone formation potential in astronauts include diets high in animal protein, frequent exercise, loss of lean body mass, and varying degrees of dehydration. Metabolic factors, so named because a change in their excretion is usually of metabolic origin, include urinary calcium, oxalate, uric acid, citrate, and pH. Risk factors that can be influenced by environmental factors include total urine volume and urinary sodium, sulfate, phosphorus, and magnesium. These metabolic and environmental factors are used to calculate physicochemical risk factors, for example, the super saturation of calcium oxalate, brushite (calcium phosphate), monosodium urate, undissociated uric acid, and struvite. A group of astronauts participated in pre- and postflight urine collections to assess renal stone risk. Average age of the 76 male and 10 female astronauts was 42.2 years. All crewmembers consumed a high-protein (>100 g/d) and high-sodium (>10 g/d) diet during their missions. Total fluid intake from foods and liquids was approximately 2 L/d or less. Crewmembers “fluid loaded” approximately 90 minutes before landing by ingesting a liter of physiologic saline. Statistically significant changes were shown for pH, calcium, and citrate in the direction of increased stone-forming risk (
2) (
Table 24-1).
Urinary citrate, a known inhibitor of renal stone formation, was lower after spaceflight (575 ± 31
mg/d) relative to before flight (707 ± 33
mg/d,
p <0.0005); this compounded the effects of increased calcium secretion. The observed decrease in mean urine volume from 1.94 ± 0.13 L before flight to 1.63 ± 0.09 L at landing (
p <0.0018) suggests that renal stone risk increased, due to concentrating urinary salts. There were some indications that length of flight increased risk also, particularly in the case of increased urinary saturation of calcium oxalate. At landing, crewmembers exhibited hypercalciuria, hypocitraturia, decreased
magnesium excretion, and decreased urinarypH and volume. A decrease in pH typically increases stone-forming potential by decreasing the solubility of uric acid and increasing the availability of uric acid crystals, which in turn can act as a nidus for calcium oxalate stones.
Another issue in regulatory physiology is associated with gastrointestinal (GI) function. The GI tract has a central role for maintaining energy balance by absorbing nutrients from food and other consumed substances in forms that the body can use. Changes in GI mobility and gastric secretion can result in decreased appetite, as well as malabsorption of amino acids, fats, vitamins, fluids, electrolytes, and many medications, which in turn affects the bioavailability of these substances. The absence of a gravity vector in spaceflight, coupled with the corresponding changes in body posture and fluid distribution, may reduce GI mobility. GI mobility has two distinct components: (a) gastric emptying (
GE) rate, which is the rate at which the stomach contents empty into the small intestine, and (b) intestinal transit time, which is the rate intestinal contents move from the small bowel to the cecum.
GE rate is known to be slower in supine subjects.
GE rate was determined in limited subjects by administering an oral dose of acetaminophen and following salivary concentrations as it is absorbed through the intestinal wall (
2); the absorption rate of acetaminophen after oral administration has been shown to be directly proportional to
GE rate. Intestinal transport time was measured using lactulose, a nondigestible sugar (one that passes undigested through the small intestine). Bacteria in the colon ferment the lactulose, producing hydrogen that is exhaled by the subject. The period between the ingestion of lactulose and peak rise in breath hydrogen represents mouth-to-cecum transit time. Both tests together permitted assessment of GI function. Preliminary conclusions from a few subjects indicated that GI function was slightly depressed in microgravity.
Artificial Gravity
Application of
AG during spaceflight has long been considered the potential “silver bullet” countermeasure. Philosophically, one could assume that removing the primary difference between spaceflight and Earth by adding appropriate levels of
AG would obviate the need for other countermeasures, with the possible exception of radiation shielding. Appropriate levels of
AG should maintain optimal physiologic function. However, several uncertainties remain as follows:
Requirements for long-arm, continuous
AG may be different from those for short-arm, intermittent
AG. Feasibility assessments have been proposed to use intermittent centripetal acceleration as
AG during spaceflight. Current proposals plan to evaluate a combination of moderate aerobic exercise with centripetal acceleration as
AG for a combined countermeasure and an initial study using
AG as a countermeasure in bed rested individuals showed promising results. There may be tradeoffs between neurovestibular risks (motion sickness, disorientation) versus the reduction in postflight deficits. Eventually, studies that examine the tradeoffs between risks and benefits of
AG must be explored with respect to bone, muscle, neurovestibular, and cardiovascular functions. These are complex investigations, and correctly meeting their inflight requirements will severely limit other biomedical activities on those missions. Nevertheless, a group of preeminent scientists recently concluded that
NASA should sponsor a rigorous, peer-reviewed research program to systematically investigate rotational
AG as a multidisciplinary countermeasure during long-duration space missions.