Primary tumor (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
T1
Tumor ≤5 cm in greatest dimension*
T1a
Superficial tumor
T1b
Deep tumor
T2
Tumor >5 cm in greatest dimension*
T2a
Superficial tumor
T2b
Deep tumor
*Note: Superficial tumor is located exclusively above the superficial fascia without invasion of the fascia; deep tumor is located either exclusively beneath the superficial fascia, superficial to the fascia with invasion of or through the fascia, or both superficial yet beneath the fascia
Regional lymph nodes (N) | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1* | Regional lymph node metastasis |
*Note: Presence of positive nodes (N1) in M0 tumors is considered stage III | |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis |
Anatomic stage/prognostic groups | ||||
|---|---|---|---|---|
Group | T | N | M | Grade |
Stage IA | T1a, T1b | N0 | M0 | G1, GX |
Stage IB | T2a, T2b | N0 | M0 | G1, GX |
Stage IIA | T1a, T1b | N0 | M0 | G2, G3 |
Stage IIB | T2a, T2b | N0 | M0 | G2 |
Stage III | T2a, T2b | N0 | M0 | G3 |
Any T | N1 | M0 | Any G | |
Stage IV | Any T | Any N | M1 | Any G |
*Grade (G) is determined by using the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system (see Table 27.2) | ||||
Table 27.2
Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) histologic grade criteria
Tumor differentiation | Mitotic count | Necrosis |
|---|---|---|
1: Well | 1: n < 10 per 10HPF | 0: No necrosis |
2: Moderate | 2: 10–19 per 10HPF | 1: <50 % |
3: Poor | 3: n ≥ 20 per 10HPF | 2: ≥50 % |
Grading of soft tissue sarcomas has important prognostic implications. Histologic grade is the strongest predictor of patient outcome. Other prognostic factors for sarcomas include primary tumor site, resection margin status, size of tumor, and primary versus recurrent disease [38, 39]. Linehan and colleagues found that tumor location significantly impacted 5-year survival among patients with completely resected liposarcomas [39]. The overall 5-year survival rate for retroperitoneal sarcomas is significantly lower compared to high-risk extremity sarcomas, 25–55 % versus 60–75 % [38]. Along with grade and tumor location, margin status also impacts survival rates. A study at Memorial Sloan Kettering Cancer Center found an 83 % disease-specific survival for margin-negative patients, compared to 75 % for margin-positive patients [40]. Margin status at the time of resection significantly impacts the rate of recurrence, which has been shown to predict future recurrences and affect survival [41]. Local recurrence rates for retroperitoneal sarcomas range from 40 to 65 % [42].
Evaluation of Extremity Sarcomas
According to the National Comprehensive Cancer Network (NCCN) guidelines, all patients with a new mass concerning sarcoma should undergo management by a multidisciplinary team [42]. The specific sarcoma workup should include a history and physical imaging of the tumor, a carefully planned biopsy, and a staging workup for distant disease [42] (Fig. 27.1).
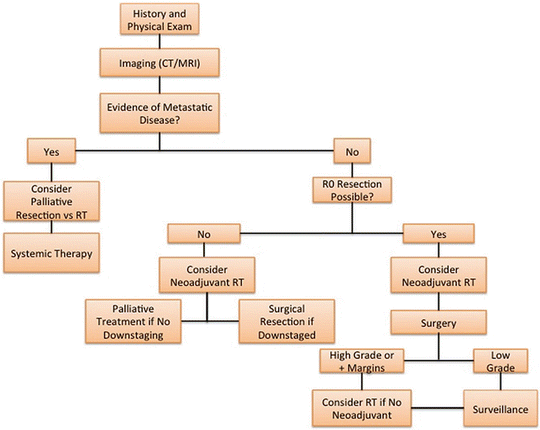

Fig. 27.1
Treatment algorithm for soft tissue sarcomas (RT radiation therapy, R0 no gross or microscopic disease)
Workup of a patient with an extremity soft tissue mass always begins with a thorough history. Important information to gather includes: time course, circumstances preceding the mass, and a thorough family history to rule out familial cancer syndromes. Patients with extremity sarcomas often present with a newly found painless mass [43]. One of the more common presentations is a recent history of trauma to the area where the mass is identified. Trauma does not necessarily cause sarcoma development, but it allows for a heightened awareness to a particular area leading to examination and imaging of that area [2]. Patients may also present with pain and neurologic symptoms at the site of the mass. After a detailed history is obtained, a full physical examination should be performed with particular focus on the mass. The mass will be more noticeable depending on whether it is deep or superficial and the body habitus of the patient. Sarcomas located below the fascia usually attain a large size prior to diagnosis. For example, a deep thigh mass is often significantly larger at the time of diagnosis compared to a more superficial mass on the forearm [44]. The size, exact anatomic location, overlying skin changes, neurological defects or muscle weakness, and vascular compromise are all important aspects of the physical exam (Fig. 27.2).
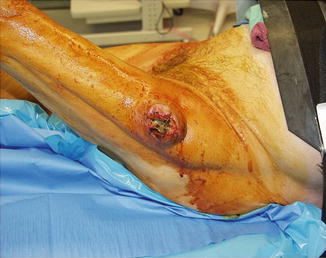

Fig. 27.2
Extremity sarcoma located on the lateral aspect of the patient’s thigh (Courtesy of Quyen D. Chu, MD, MBA, FACS)
The next phase in an extremity sarcoma workup is to image the mass. Imaging allows for treatment planning prior to surgical resection by determining its size, location, association with surrounding structures, and potentially even histologic information. Imaging modalities include conventional radiography, ultrasound, computed tomography (CT) scan, and magnetic resonance imaging (MRI) (Fig. 27.3); each of these modalities has their relative merit and downsides. Conventional radiography provides good resolution of the bone trabecular, therefore providing information regarding bony involvement of the mass [45]. Ultrasound is helpful in determining if the mass is cystic or solid. It also helps guide tissue biopsy. CT provides cross-sectional imaging with the benefits of rapid image acquisition, decreased cost compared to MRI, and excellent bony and solid organ detail [45]. It can also be utilized when MRI is contraindicated. The addition of intravenous contrast allows for further determination of the vascularity of the tumor, the tumor size and location, and its anatomic association with large arteries and veins [46]. CT should also be used in the initial staging workup and in follow-up for the detection of metastatic disease in the lungs and liver, particularly for high-grade tumors. The issues with CT imaging include exposure to radiation, risk of allergic reactions to the contrast dye, and risk of contrast-induced nephropathy. MRI is the imaging modality of choice for soft tissue sarcomas of the extremity. This modality gives precise anatomical definition, revealing the relationship of the tumor to the investing fascia, size of the tumor, potential histologic characteristics, and proximity to neurovascular structures [47]. MRI can also be used to determine the enhancement pattern and amount of necrosis that can be followed during treatment [48]. The benefits of MRI include the lack of radiation exposure; problems include long acquisition time, increased expense, the contraindication in patients with metal implants, and anxiety in patients with claustrophobia [45].
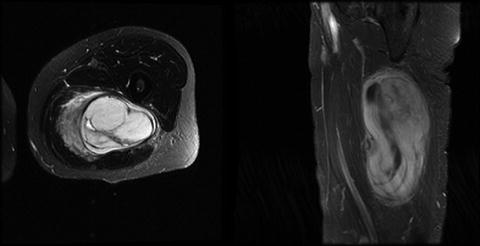

Fig. 27.3
MRI of a patient with a malignant fibrous histiocytoma (MFH) of the medial thigh. The patient underwent preoperative chemotherapy followed by a complete resection with a negative margin greater than 1 cm. Final pathology demonstrated no residual tumor (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Once imaging has been completed, tissue biopsy may be indicated for tissue diagnosis. There are two ways to obtain tissue for a pathological diagnosis: needle biopsy and surgical biopsy. Needle biopsy can be divided into fine needle aspiration (FNA) and core needle biopsy. Both of these techniques can be done with and without radiological guidance. In the setting of extremity sarcoma, ultrasound is typically the radiologic guidance of choice. FNA is performed using a small gauge needle, passing it through the lesion multiple times on negative pressure. The downside of FNA is that it does not provide tissue architecture and supplies only a limited number of cells for evaluation and specialized pathologic investigations. Core needle biopsy is performed using a specialized biopsy needle that removes a core of tissue. This technique preserves the architecture of the tissue and typically provides enough cells for specialized immunostains while remaining minimally invasive. For these reasons, core needle biopsy has become the biopsy technique of choice [49]. When performing needle biopsy, the needle should enter through the proposed incision for resection on the skin so that the needle tract can be excised with the specimen. This eliminates the chance of local recurrence at the biopsy site [50]. Some have advocated tattooing the needle biopsy site to ensure removal at final resection.
The other choice for obtaining tissue is through an excisional or incisional surgical biopsy. Excisional surgical biopsy is an inadequate method for performing a biopsy on a mass concerning for sarcoma because a margin is not taken. In the setting of sarcoma, a second operation would need to be performed to obtain appropriate surgical margins. Incisional surgical biopsy is a reasonable approach when access to core needle biopsy is unavailable. To minimize recurrence at the biopsy site, a small incision along the direction of the proposed resection is performed.
Management of Primary Extremity Sarcoma
The mainstay of management of extremity sarcoma is surgical resection with negative margins [43]. This type of local control was historically obtained through amputation of the affected limb causing severe functional and psychological ramifications to the patient. Regardless, amputation was allowed for good local control and was the treatment of choice for decades [51]. In 1982, a prospective randomized clinical trial from the National Cancer Institute was published comparing limb-sparing surgery with radiation versus amputation for extremity sarcoma. The results showed no difference in 5-year overall survival rates between the two groups, and there was a nonsignificant increase in local recurrence rates in the limb-sparing surgery with radiation group [52]. These data support the use of limb-sparing surgery for the treatment of extremity sarcoma. Despite these results, amputation may still be the preferred choice of treatment depending on the involvement of major neurovascular structures and potential functional outcome after treatment.
The ultimate goal of resection includes complete removal of the tumor including the biopsy site and tract, while preserving a functional limb. An important aspect of limb-sparing resection is to ensure that negative margins are obtained. A number of studies have shown that gross and even microscopic margins lead to an increased risk of local recurrence [44, 53]. Although the prognostic significance of a positive margin is clear, the question remains what should a surgeon do if faced with a positive margin after resection. A retrospective review from MD Anderson Cancer Center of patients with positive margins after surgery showed that those who underwent re-resection to negative microscopic margins had a better local control than those who did not, despite the use of postoperative radiation therapy [54]. Therefore, it is recommended that a re-resection be preformed, if anatomically possible, to obtain negative margins. When performing these oncologic resections, it is important to make incisions longitudinally along the limb so that the mass may be resected with the best chance of primary closure. If the patient did not have preoperative radiation, it is important to keep the drains near the surgical field so they can be incorporated within the radiation field. If a large surgical defect is expected after resection, reconstruction planning is an important aspect of the perioperative surgical care.
For the most part, there is no role for lymph node sampling/dissection because the risk of regional metastasis is ≤5 %. However, it should be considered for patients with synovial sarcoma, epithelioid sarcomas, clear cell sarcomas, vascular sarcomas, and rhabdomyosarcomas since lymph node metastasis can occur in up to 24 % of patients [55].
Radiation therapy is used to improve local control when combined with limb-sparing surgery. A study from the National Cancer Institute highlighted the role of radiation therapy in a study randomizing patients undergoing limb-sparing surgery for sarcoma to receive postoperative radiation or no postoperative radiation. In patients with either high-grade sarcomas or low-grade sarcomas, there was a statistically significant improvement in local control if postoperative radiation was received. In this study, there was no difference in the overall survival between the two groups [56]. This study highlights the importance of radiation in the local control of sarcoma for patients undergoing limb-sparing surgery. External beam radiation has been the delivery method of choice; however, some groups have studied brachytherapy and intraoperative radiation therapy with and without external beam radiation, with good local control rates [57].
After surgical resection, the radiation field for external beam radiation can be quite extensive. There are also theoretical and logistical benefits associated with neoadjuvant radiation therapy [58]. Radiation doses in the preoperative setting tend to be lower, and the radiation field tends to be smaller if performed preoperatively. There is a decrease in long-term radiation effects, such as fibrosis and limb/joint dysfunction, if the radiation is given preoperatively [59]. In a prospective randomized study of preoperative radiation versus postoperative radiation in extremity sarcomas, there was no difference in local control rates between the two groups. There was also no difference in the margin status after the resection between the two groups. There was, however, an increase in the wound complication rate: 17 % for the postoperative radiation group compared to 35 % for the preoperative radiation group. Preoperative radiation also resulted in an increased rate of secondary wound closures (grafts and flaps). However, preoperative radiation was associated with decrease toxic skin effects from radiation, as compared to postoperative radiation [60]. The utility of radiation in a subset of patients with small or low-grade tumors remains controversial and deserves further investigation. According to the NCCN guidelines for stage I tumors with resection margins >1 cm, it is acceptable to omit radiation [5, 42].
Despite the good local control obtained from surgery and radiation therapy, there are a significant number of patients with high-grade sarcomas that will develop metastatic disease. The role of adjuvant chemotherapy with the goal of decreasing metastatic rates in primary sarcoma is uncertain. Most chemotherapy regimens have included Adriamycin, with well-defined toxicities. A meta-analysis of randomized trials showed an improvement in time-to-local and distant recurrence for those who received chemotherapy, but no improvement in overall survival [61]. The European Organization for Research and Treatment of Cancer concluded a multicenter randomized trial (EORTC 62931), in which patients with resected soft tissue sarcomas were randomized to receive a chemotherapy regimen or no chemotherapy. The trial showed no difference in overall survival (66 % vs. 67 %) or relapse-free survival between the two groups [62]. Given the unclear benefits of adjuvant chemotherapy, patients at high risk for metastatic disease should consider a discussion of the risks and benefits to chemotherapy with a medical oncologist.
The neoadjuvant approach to chemotherapy in sarcoma has been studied and may be even more controversial than the adjuvant approach. A single institution review of neoadjuvant chemotherapy alone in high-grade extremity sarcomas failed to show improvement in local control or overall survival [63]. Radiation has been added to the chemotherapy regimens in an attempt to improve both local control and decrease distant metastasis. A single institution review of this approach has shown that it has good long-term survival and tolerable toxicities, but there has yet to be a randomized study comparing the different approaches [64]. Another group is looking at the role of regional hyperthermia at the time of neoadjuvant chemotherapy for extremity sarcomas. In a prospective randomized multicenter trial comparing neoadjuvant chemotherapy to neoadjuvant chemotherapy with hyperthermia, they found that patients who had hyperthermia had improved local recurrence and disease-free survival [65]. Despite this interesting work, there is little data comparing an adjuvant approach for chemotherapy to a neoadjuvant approach so patients who are interested in this approach should consider clinical trials.
It is important to address the management of patients who present with synchronous metastatic disease, a clinical scenario that occurs in 12–23 % of patients with sarcomas [66, 67]. Kane et al. reported the outcome of 48 patients from a single institution who presented with synchronous metastases and treated with aggressive surgical resection of both the primary and metastatic sites [68]. Pulmonary metastases were most commonly encountered (n = 30), followed by nodal (n = 11) and liver (n = 4) disease. Seventeen patients underwent resection of their metastatic site. Although the median survival for patients undergoing metastasectomy was slightly longer than that of patients with unresectable disease, the difference was not statistically significant. The authors concluded that resection of soft tissue sarcoma and synchronous metastases should be approached with caution and reserved for patients requiring symptomatic palliation [68].
Follow-Up and Surveillance
Once treatment has been completed, patients enter the surveillance phase. The goal of surveillance is to identify local regional recurrence or metastatic disease while it is still treatable. The clinical follow-up and surveillance of extremity sarcoma patients include a combination of history, physical examination, and imaging. Most recurrences occur within the first five years of initial treatment; therefore the surveillance schedule is more intense in the early phase and decreases after five years. It is recommended that a history and physical examination focusing on the local regional area of resection should be done every 3 months for the first 3 years, every 6 months for years 4–5, and annually after 5 years. There are no specific lab tests that are needed secondary to the lack of tumor markers available for this disease. MRI of the primary site should be obtained to look for local regional recurrence every 6 months for the first 3 years, annually from years 4–5, and only if suspected after year 5. If the patient has contraindication to MRI, then CT should be performed of the primary site. CT imaging of the chest in patients with high-grade sarcomas to rule out lung metastases is also important, with images recommended every 6 months for the first 3 years, and annually during years 4–5. Patients should also perform self-examinations on a regular basis at home and alert their treatment team if there are any changes. The above recommendations comply with the NCCN guidelines for surveillance of sarcoma patients [42].
Management of Recurrent and Metastatic Extremity Sarcoma
Extremity sarcomas may recur locally or at distant sites. A number of groups have reviewed prognostic factors that increase the likelihood of a local recurrence. There is a consensus that a positive margin and a recurrent sarcoma are both independent prognostic factors for local recurrence [69, 70]. The unfortunate event of locally recurrent extremity sarcoma provides significant challenges to the treatment team. If the recurrence was detected on physical examination, imaging is important to delineate relationship to surrounding anatomic structures and vasculature. A metastatic workup should also be done with CT scan of the chest to rule out synchronous metastasis. It is also important to obtain all prior clinical documentation (treatments, pathology) for treatment planning [71]. In patients with isolated local recurrence and no prior history of radiation treatments, it would be appropriate to consider preoperative radiation followed by surgical resection or surgical resection with adjuvant radiotherapy. If the patient has already received radiation, the options after re-excision include limited additional external beam radiotherapy, brachytherapy, or intraoperative radiation therapy. Isolated limb perfusion can be considered at centers conducting clinical trials. Surgically aggressive limb-sparing therapy can lead to very good local control rates. In a single institution retrospective review, 87 % of patients obtained durable local control with or without re-irradiation [72].
In addition to local recurrence, patients may also develop distant metastases. 20–40 % of patients with extremity sarcoma will have or develop pulmonary metastases. In a review from Memorial Sloan Kettering Cancer Center, the only prognostic factor that determined good outcome in patients with sarcoma metastatic to the lung was complete resection [73]. In a more recent retrospective review of 48 patients with metastatic sarcoma to the lung that had undergone pulmonary resection, the 5-year overall survival rate was found to be 52 % [74]. These data highlight that careful selection of patients combined with an aggressive approach in resection of pulmonary metastases can result in favorable outcomes.
If resection is not possible, the efficacy of chemotherapy regimens is currently limited. The standard doxorubicin-based regimens have modest clinical efficacy and significant treatment-associated toxicities [75]. However, newer regimens show promise in early phase testing. In a phase II trial of gemcitabine and docetaxel compared to gemcitabine alone, there was an increase in both progression-free survival and overall survival. Unfortunately, the combined regimen was also quite toxic [76]. Trabectedin, a marine-derived antineoplastic compound, has also shown promise in treating metastatic liposarcoma and leiomyosarcoma [77].
Evaluation of Retroperitoneal Sarcomas
Patients with retroperitoneal sarcomas (RPS) commonly present with symptoms of abdominal fullness or obstruction involving the gastrointestinal or renal systems. Pain, neurologic symptoms, and weight loss are other common presenting symptoms. Symptoms are initially vague in nature, precluding many patients from seeking early medical attention. The relatively non-confining space of the retroperitoneum can conceal a mass for an extended period of time, allowing tumors to become quite large prior to the development of clinical symptoms.
Patients who present with suspicious symptoms of an abdominal mass should undergo imaging with a CT of the abdomen and pelvis (Fig. 27.4). A CT chest is performed to complete the staging workup and evaluate for metastatic disease. A well-described fatty tumor of the retroperitoneum without hyperdense areas is considered a well-differentiated lipomatous tumor or well-differentiated liposarcoma. Both diagnoses require surgical resection and biopsy is not usually indicated. A lipomatous tumor of the retroperitoneum with higher dense areas is diagnostic for retroperitoneal liposarcoma. The higher dense areas may represent areas of dedifferentiation or simply areas of different liposarcoma histologic subtypes. Image-guided biopsy is indicated if therapy other than surgery is considered as first-line treatment [78]. Retroperitoneal masses with little or no fatty component have a large differential diagnosis that includes germ cell tumors, lymphoma, abscess, renal and adrenal tumors, neurogenic tumors, undifferentiated carcinoma of primary or metastatic origin, and sarcoma. Because of the extensive differential diagnosis, most authors advocate for an image-guided tissue biopsy in these tumors. Any soft tissue mass in an adult that is larger than 5 cm, growing or persistent beyond 4–6 weeks, that is not characteristic of a liposarcoma should be biopsied [6]. FNA and core biopsy can both be used for diagnosing soft tissue sarcomas. Core biopsy is 95 % accurate in diagnosing sarcomas, whereas the accuracy of FNA biopsy is largely dependent on the experience of the cytopathologist interpreting the results [41]. One advantage of core biopsy over FNA is that cores provide tumor architecture, and this allows for identification of histologic subtype and grading. The benefit of assessing tumor grade has made core biopsy the first choice for biopsy techniques. Biopsies should be approached from the retroperitoneum and should avoid the peritoneal cavity to decrease contamination with tumor cells. Additional imaging such as high-resolution CT or MRI may be indicated to help plan surgical approach and better delineate involvement of the muscles, nerves, vessels, bone, and vertebral foramen. If resection includes removal of a kidney, a preoperative nuclear renal function scan is indicated to assess the function of the remaining kidney. The only lab work indicated is the usual preoperative labs of electrolytes, coagulation studies, hemoglobin, and platelet count. Currently there are no serum tumor markers available for soft tissue sarcomas.
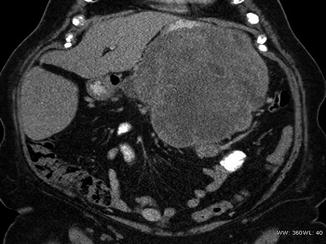

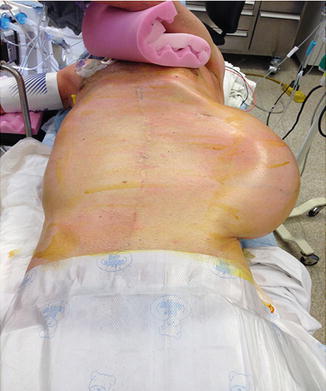
Fig. 27.4
Retroperitoneal sarcoma: large mass suspicious for a retroperitoneal sarcoma (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Management of Primary Retroperitoneal Sarcomas
A multidisciplinary approach involving surgical oncology, medical oncology, and radiation oncology is crucial to the management of these tumors. Treatment requires thoughtful planning and timing of different modalities to offer the best functional and oncologic outcomes for patients.
As in extremity sarcomas, surgical resection with negative margins is the standard of care and primary treatment for localized retroperitoneal sarcomas (RPS). En bloc resection of involved surrounding organs may be necessary to obtain clear margins. Resection of the colon, mesocolon, and muscle as part of the en bloc resection is well tolerated with limited consequences. The resection of a kidney, spleen, or distal pancreas as part of the en bloc resection has minimal short-term morbidities, and the removal of such organs is usually tolerated (Fig. 27.5). When the en bloc resection involves the head of the pancreas, duodenum, major vessels and nerves, or the liver, complication rates can be significant, and resection of such organs is usually not performed unless there is macroscopic involvement [78]. Complete negative margin resections of retroperitoneal sarcomas have been reported between 50 and 67 % [79]. Obtaining negative microscopic margins is difficult due to the extremely large mass of tumors, the extent of surface area involved, and the anatomic constraints of tumor location in the retroperitoneum. Although achieving negative margins is difficult, it has been illustrated that the patient who obtain negative margins has better outcomes [38]. Complete surgical resection improves median survival in patients with primary retroperitoneal sarcomas [80]. The 5-year survival rate for patients with complete surgical resection and R0 margins is 103 months compared to 18 months for incomplete surgical resection and positive margins [42, 81]. Surgery remains the mainstay of treatment for patients with locally resectable disease.
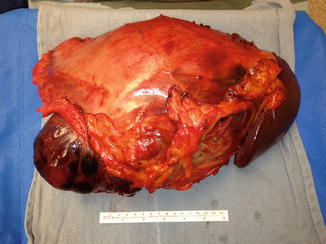

Fig. 27.5
Gross specimen of a retroperitoneal sarcoma. Patient underwent a total gastrectomy, distal pancreatectomy, and splenectomy for a large retroperitoneal leiomyosarcoma (Courtesy of Quyen D. Chu, MD, MBA, FACS)
The exact role of radiation therapy in retroperitoneal sarcoma is difficult to define. Radiation has a well-established role in extremity sarcomas; however, the adjacent radiosensitive structures in the retroperitoneum limit the use of this modality in retroperitoneal sarcomas. Single institutional studies have shown an improvement in local control rates with the addition of radiation therapy to surgery for retroperitoneal sarcomas [82, 83]. The advantages, disadvantages, and feasibility of preoperative, intraoperative, and postoperative radiation therapies are based on observational and retrospective studies [6]. Preoperative radiation allows for displacement of adjacent structures by the large tumor mass, limiting exposure of other critical structures (i.e., small bowel) to radiation thus decreasing the rate of toxicity. Preoperative radiation also clearly establishes boundaries of the intended treatment fields. Postoperative radiation on the other hand allows for better assessment of tumor histology and grade, which enables selection of patients most likely to benefit from radiation therapy. However, when the large tumor is removed, a void is created and filled with other critical structures. These structures are exposed to the radiation, resulting in increased gastrointestinal toxicity with postoperative radiation therapy. The lack of level 1 evidence and the varied radiation treatment plans in published series make discernment of the timing of radiation in retroperitoneal sarcomas difficult to determine. Preoperative radiation therapy with complete surgical resection has been associated with improved recurrence-free survival, disease-specific survival, and overall survival. The 5-year local recurrence-free survival of 60 %, disease-specific survival of 46 %, and overall survival of 61 % were demonstrated in two prospective trials of patients with intermediate and high-grade retroperitoneal sarcomas who had preoperative radiation therapy and aggressive surgical resection with R0 or R1 status [84]. Postoperative radiation has been associated with improved relapse-free survival but has not impacted overall survival [85, 86]. The addition of intraoperative radiation therapy to aggressive surgical resection and preoperative external beam radiation improves local recurrence-free rates to 83 % from 61 % and overall survival rates to 74 % from 30 % when compared to surgical resection alone [87, 88]. At our institution, preoperative radiation with or without intraoperative radiation has become the standard approach to RPS. Published data from our institution has shown one- and two-year local control rates of 64 and 50 % and OS of 90 % at 1 year and 74 % at 2 years with minimal toxicity from preoperative radiation [58].
Current recommendations state that for all patients with surgically resected disease and R1 margins who did not receive preoperative radiation should undergo postoperative radiation [42]. Radiation should also be considered in patients with an R0 resection and high-grade tumors [42]. Patients who received preoperative radiation and underwent surgical resection with R1 margins may receive an additional radiation boost to the tumor bed [42].
The role of chemotherapy for localized soft tissue sarcomas of the retroperitoneum is limited. Most commonly, chemotherapy for localized disease is used as a radiation sensitizer or as neoadjuvant cytoreductive therapy to improve achievement of an R0 resection. A recent meta-analysis by Pervaiz et al. found a marginal benefit to adjuvant chemotherapy with respect to local recurrence, distant recurrence, overall recurrence, and overall survival [89]. Their work demonstrated an odds ratio (OR) for local recurrence of 0.73 (95 % CI 0.56–0.94; p = 0.02) and an OR for overall survival of 0.56 (95 % CI 036–0.85; p = 0.01) in favor of adjuvant chemotherapy. The greatest benefit was achieved with a combination-based chemotherapy of doxorubicin and ifosfamide [89].
Follow-Up and Surveillance
Local recurrence is a major morbidity and a leading cause of mortality in patients with retroperitoneal sarcomas. Because 40–60 % of patients with retroperitoneal sarcomas will develop a recurrence, close follow-up is vital to improving patient outcomes [42].
According to the NCCN guidelines, patients who undergo surgical resection and have an R0 margin status should have a history and physical exam and CT of the abdomen and pelvis every 3–6 months for 2–3 years and then annually. Patients who undergo surgical resection and have an R1 margin status or have a high-grade tumor with an R0 margin status should have a history and physical exam and CT of the abdomen and pelvis every 3–6 months for 2–3 years, then every 6 months for 2 years, then annually.
Management of Recurrent and Metastatic Retroperitoneal Sarcomas
Recurrence is common with retroperitoneal sarcomas. 40–60 % of resected retroperitoneal sarcomas will develop recurrence. Each local recurrence makes complete surgical resection more difficult and less likely.
Recurrent retroperitoneal sarcomas are evaluated with a biopsy and staged with CT imaging of the chest, abdomen, and pelvis (Figs. 27.7 and 27.8). Once metastatic disease is ruled out and the recurrence is determined to be local, the patient should undergo complete surgical resection with a curative intent, if feasible (Figs. 27.6, 27.7, 27.8, 27.9 and 27.10). The success for complete resection is favorable with the first recurrence but significantly decreases with each additional recurrence. Lewis et al. showed that 57 % of patients with a first local recurrence were able to undergo complete surgical resection where only 22 % of second and 10 % of third recurrences were completely resected [81].