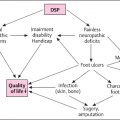
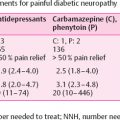
6 Socioeconomic Aspects
 Direct Costs of Diabetic Neuropathy
Direct Costs of Diabetic Neuropathy
A substantial proportion of the economic burden of diabetes is attributable to its complications, in particular, cardiovascular disease. In the United States, direct medical costs attributable to diabetes in 1997 totaled $44.1 billion, including $11.8 billion (27%) for excess prevalence of diabetes-related chronic complications [1]. The major cost factors were cardiovascular disease (17.2%), followed by neurologic (3.4%) and renal (2.3%) complications.
Costs of Medical Care For Diabetic Neuropathy
There are few cost-of-illness studies assessing the economic impact of diabetic neuropathy. The American Diabetes Association estimated $1.5 billion total health care expenditures attributable to diabetes for treatment of neurologic diseases in the US in 1997 [1]. The most cost-intensive health care sector was inpatient care, accounting for about 70% ($1.0 billion), whereas outpatient treatment comprised only 8% ($119 million). Neurologic disorders included not only peripheral neuropathy, neuralgia, and neuritis, but also cerebrovascular disease, transient ischemic attacks, and stroke, which are major cost factors.
Based on the US 1987 National Hospital Discharge Survey, diabetic polyneuropathy, mononeuropathy, amyotrophy, and peripheral autonomic neuropathy alone contributed to about 36 000 hospital admissions with an average length of stay of 8.6 days and average costs per day of $596 [2]. Total inpatient costs for these disorders in the US were estimated to amount to $184 million in 1987. Costs for inpatient treatment of diabetic foot problems were not given separately in this analysis.
In an earlier study based on US national data from 1980, health care expenditures for treatment of neuropathy in type 2 diabetic patients comprised 2% ($240 millions) of total costs attributable to diabetes [3]. Hospital treatment was found to be the major factor, contributing to about 90% of costs. Expenditures for chronic skin ulcers, which are closely related to peripheral neuropathy, were estimated to amount to $145 million in the type 2 diabetic population.
Using data from a large health maintenance organization in the US. Selby et al. estimated excess costs of hospitalization for disorders of the peripheral nervous system in diabetic patients compared to an age-and sex-matched nondiabetic control group [4]. Diabetic patients were 1.5-fold more likely to be hospitalized for treatment of neuropathy, yielding annual excess costs of $137 000 among these 85 000 patients, which was a minor cost factor (< 0.01 % of total excess costs) compared to other complications, in particular cardio-vascular and end-stage renal disease.
In the UK, Williams estimated that about 13% of total costs of care for diabetic patients could be attributed to peripheral neuropathy and vascular disease [5]. In another British study, nationwide direct health and social care costs in the type 1 diabetic population were estimated as US$157 million in 1992 [6]. Neurologic complications, which were not further specified, were reported to comprise 1.2% ($2.0 million), almost completely due to hospital treatment.
More recently, expenditures of inpatient care for diabetic neuropathy in the United Kingdom were estimated. From 1991 to 1995, routine hospital data from a large population of more than 400,000 people, whose demographic characteristics largely reflect those of the UK, were analyzed for admissions for mononeuritis and inflammatory and toxic neuropathy [7]. Diabetic patients comprised 7.8% of all subjects with one of these admission diagnoses, accounting for 12.2% ($151 346) of total hospital costs for these disorders over a study period of four years. There are no comparable comprehensive analyses of the costs of diabetic neuropathy in other health care systems.
In conclusion, cost-of-illness studies on diabetic neuropathy suggested that nerve disorders contribute directly to only a small proportion of total health care costs (about 2%) in the diabetic population, mostly due to hospital treatment. However, most of these analyses were based on the International Classification of Diseases (ICD) coding of principal diagnoses, and the attributable costs of neuropathy in diabetic foot complications were not included, which has probably led to an underestimation of the real economic impact of diabetic neuropathy.
Costs of Medication
Overall, prescription drug costs in people with diabetes have been found to be about three-fold higher than in nondiabetic patients [8]. The most important cost factors were cardiovascular drugs and antidiabetic agents. Compared to these drug groups, pharmacologic treatment of neuropathy was a minor cost factor. Nationwide prescription drug costs in German primary care practices attributable to diabetic neuropathy were estimated to amount to US$61 million in 1996, which was about 1.4% of total estimated annual drug costs in diabetic patients [9]. It is noteworthy that about one-third of these medication costs were due to drugs without proven effectiveness in symptomatic diabetic nerve disorders (e.g. vitamin B, vasodilator drugs). These results indicate the urgent need for cost-effective drug treatment guidelines for symptomatic diabetic neuropathy. Furthermore, economic studies assessing the cost-effectiveness or cost-utility of drug treatment of diabetic neuropathy are currently lacking.
Potential Cost Savings by Prevention of Diabetic Neuropathy
The effect of intensive diabetes therapy on the incidence of neuropathy in type 1 diabetes was estimated using a simulation model based on data from the Diabetes Control and Complications Trial (DCCT) [10]. The cumulative incidence of neuropathy was predicted to be 31 % at age 70 years in people receiving intensive therapy, compared to 57% with conventional treatment (46% reduction). The predicted total lifetime costs per type 1 diabetic patient for intensive insulin therapy were $99 822, which was, on average $33 746 more than for conventional insulin therapy. The model estimated an overall average gain of 15.3 additional years of life free from any significant microvascular or neurologic complication and a 5.1-year increase in survival. When length of life was adjusted for quality of life using utilities for blindness, end-stage renal disease, and lower extremity amputation, the incremental cost per quality-adjusted life year (QALY) gained under intensive insulin therapy was $19 987, which was within the range of other accepted therapeutic interventions in chronic diseases.
A similar probabilistic model was used for type 2 diabetic subjects, assuming that the effects of intensive diabetes treatment observed in type 1 diabetes (hazard rate for complications) can be applied [11]. A lifetime incidence for symptomatic distal neuropathy of 10% under intensive care was estimated, compared to 31 % under standard treatment (reduction: 68%). The cumulative incidence of lower extremity amputations also decreased from 15% to 5% (a drop of 67%). Therefore, under intensive care, the predicted average lifetime costs per type 2 diabetic person for neuropathy and lower extremity amputation were $1469 compared to $4381 under standard treatment (difference: $2912). A small randomized clinical trial in middle-aged type 2 diabetic patients in Japan (Kumamoto study) indicated a 60% risk reduction for clinical neuropathy over ten years in subjects receiving intensified versus conventional insulin treatment, yielding 2.2 additional years free of this disorder [12]. Total average direct costs for microvascular, neuropathic, and macrovascular complications combined in the trial patients (hospitalizations, drugs, ophthalmic treatment) were about 50% lower in the intense-treatment group (conventional: $15 565; intense: $7591). Thus, improved glycemic control carries a considerable cost-saving potential with respect to neurologic complications of diabetes.
Methodologic Considerations
There are no representative studies estimating the cost distribution of the various medical problems related to diabetic neuropathy, e.g., paresthesia and pain, neuropathic foot ulcer and amputation, or autonomic dysfunction. It has been stated that this may be a result of the disparate way in which diabetic patients with neuropathy and neuropathy-related complications use health services, e.g., episodes of lower limb complications spread over many different clinical specialties. This points to another methodologic problem in assessing neuropathy-related costs: different specialists will use different diagnostic terms for the same clinical problem, making valid comparisons or data aggregation difficult. Furthermore, in some cases the link between neuropathy and specific symptoms or disorders will be less evident (e.g., autonomic dysfunction of the gastrointestinal or genitourinary tract). Thus, previous studies most likely largely underestimated the economic impact of nerve disorders in diabetes.
 Costs of Foot Ulcer and Lower-Extremity Amputation
Costs of Foot Ulcer and Lower-Extremity Amputation
Neuropathy is the major risk factor leading to foot ulceration and lower extremity amputation. Most previous cost-of-illness studies of diabetic foot ulcers and amputations have only estimated direct costs (hospitalizations, procedures, wound care, etc.). Only a few of these economic studies have been performed outside the US (e.g., Sweden, The Netherlands, UK). It is therefore important to note that economic estimates are not applicable to other health care systems and may even not be valid for the same geographic region at different periods (e.g., change in reimbursement, move from acute care to rehabilitation).
Cost-of-Illness Studies: Data Sources
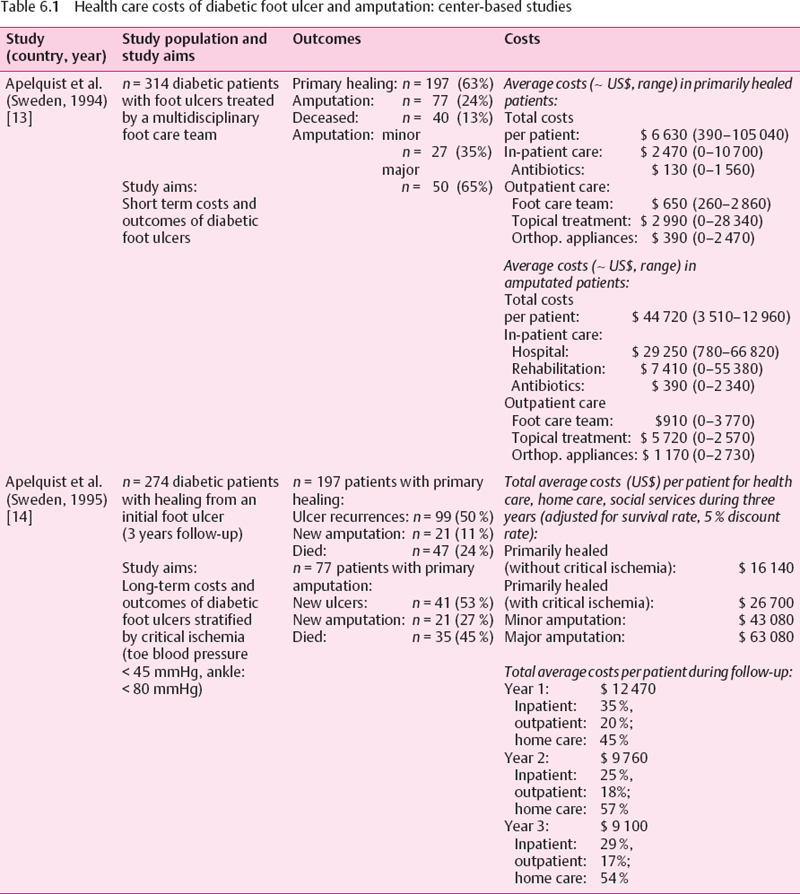
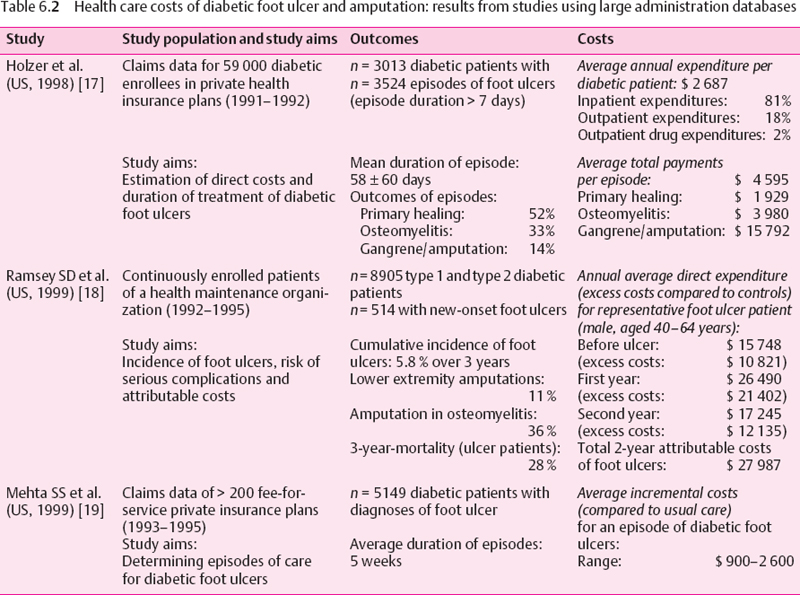
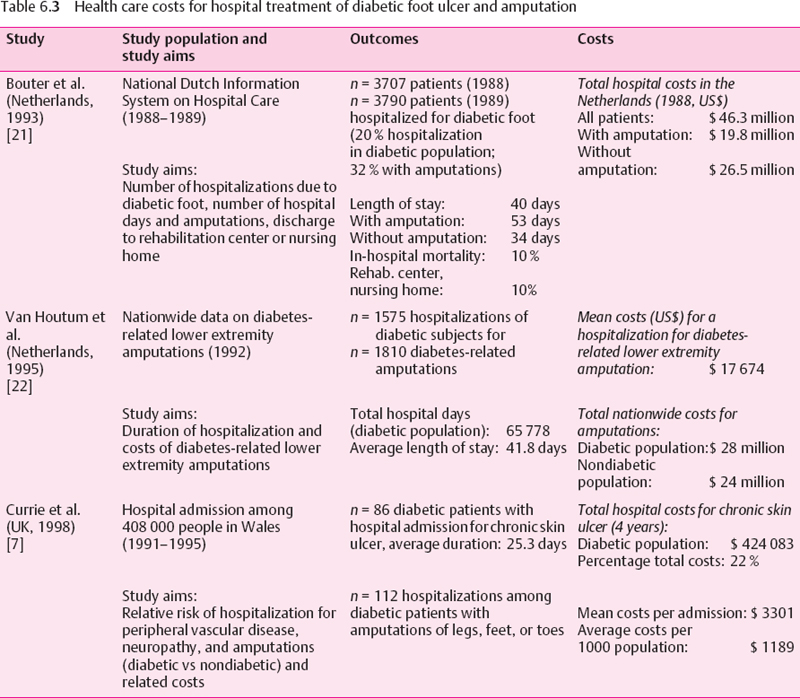
A variety of study approaches have been used to estimate the health care costs of diabetic foot complications. The strengths and weaknesses of the various study designs need to be considered. Center-based studies (e.g., diabetic foot clinics) allow a comprehensive assessment of the total costs in a well-defined population: however, a referral bias towards more severe cases is likely. Large automated databases (health maintenance organizations, private insurance companies) facilitate detailed assessment of procedures in large patient populations. However, diagnoses usually have low specificity (e.g., ICD coding for foot ulcer) and are restricted to persons enrolled in these health insurance plans, who are often younger, healthier, and higher educated than the general population. National data on hospital admissions for diabetic foot complications or amputations are often incomplete and comprise only one cost component. Finally, economic models (decision analyses, Markov models), which have been used to predict long-term economic consequences, depend on the quality of the underlying epidemiologic data. Unfortunately, there are few prospective studies of long-term outcomes of diabetic foot complications. The main results of these studies are summarized in Tables 6.1–6.3.
Center-Based Studies
In Sweden, Apelquist et al. have found that in diabetic patients in whom primary healing of their foot ulcer could be achieved, outpatient costs were the major cost factor, comprising about two-thirds of total expenditures [13]. As expected, in patients requiring amputations, inpatient costs were more important (83% total costs), leading to about seven-fold higher total average costs than in primary healing. It is noteworthy that in both groups (healed ulcer and amputations) an enormous range and overlap of costs was observed, depending on the individual clinical course of the patients (Table 6.1). This should be kept in mind when average costs for treatment of foot complications are calculated, e. g., for reimbursement. Costs in patients with primary healing increased with the severity of the lesions in the Swedish study, e. g., average total costs in patients with deep ulcerations were roughly three-fold higher than those for treatment of superficial ulcers. Thus, from an economic perspective, too, early intervention in foot ulcerations is mandatory. Other risk factors for increased health care costs were a healing time of more than two months and the presence of impaired peripheral circulation.
In the group requiring primary amputation, total costs for patients with minor amputations (US$33 540) were only about two-third of the costs for subjects who underwent major amputations ($50 700), mainly because hospital stays were shorter. It is further noteworthy that average costs were substantially higher for minor amputations than for subjects with primary ulcer healing without amputation, which was due to the longer wound healing periods in this population.
In the same cohort of diabetic patients, the long-term costs over a period of three years were assessed (Table 6.1) [14]. Long-term costs for patients with initial primary healing were higher in subjects with critically impaired peripheral circulation, which was mainly due to a higher incidence of new ulcerations. In both patients with initial minor and major amputation, health care costs were substantially higher, which was explained by cost-intensive inpatient care especially during the first year of follow-up. In long-term care, home care and social services became the major cost factors. High expenditures also occurred because of recurrent ulcers and amputations in this high-risk population with a poor prognosis. Almost half of the patients died within the three-year follow-up period.
Maximum limb salvage in patients with severe diabetic foot complications (ischemia, sepsis) can be achieved by prolonged hospital treatment including surgical revascularization. In the United States, at the New England Deaconess Hospital in Boston, the incidence of amputations fell from 44% to 7% from 1984 to 1990 as the result of an intensive multidisciplinary approach [15]. Length of hospital stay, average bypass graft costs (1984: $19 470; 1990: $15 796), and average costs of amputations (1984: $20 248; 1990: $18 341) all decreased over this period. Although quality of care improved and the overall costs decreased, the Medicare reimbursement for foot complications was insufficient, resulting in an overall loss of about $7500 per admission.
Resource use over one year was assessed among 151 patients from a diabetic foot clinic in Belgium, representing the whole range of severity of foot problems [16]. Mean annual costs per ulcer was $5227. The most important cost contributor was inpatient treatment (72%), followed by drugs (11%). The 16 (11%) severe wounds requiring amputations and prolonged hospitalization yielded average mean costs of $31 716, or 80% of total expenditures. Thus, the severity of foot problems determines the costs of treatment. The overall costs of diabetic foot care were largely due to prolonged hospitalization and amputations. There appears to be a shift in major cost areas over time. For patients not requiring amputation, expenses were mainly related to outpatient care. When amputation was required, inpatient care became the major cost factor.
Databases (Health Insurance Companies)
In the US, Holzer et al. have found claims for foot ulcers in 5% of a working-age population (18-64 years) of privately insured subjects with diabetes diagnoses (Table 6.2) [17]. The average costs per ulcer period of $4 600 were also mainly due to hospital treatment (80%). Mean costs increased depending on the peripheral vascular status of the patients and the severity of the ulcers. Due to methodologic limitations, the actual magnitude of foot ulcer costs is most likely higher than estimated in this study.
In another study using data from a large health maintenance organization, Ramsey et al. observed an annual incidence of about 2% of diabetic foot ulcers [18]. Excess care costs during the first year, estimated as the difference of total mean costs compared to subjects without foot ulcer, were substantial (e.g., in males aged 40-64 years: $21 400). The attributable costs for care of diabetic foot ulcers during the first two years after diagnosis were estimated to amount to $28 000 per average foot ulcer patient. Health care costs were already increased prior to the diagnosis, which may be related to comorbidities and diagnostic procedures.
Another economic study, using data from US private health insurance plans, estimated a duration of five weeks for an average episode of care for a diabetic foot ulcer, with an estimated range from 1 to 13 weeks [19]. The costs of care for one episode ranged from $900 to $2600.
Using Medicare claims data from 1995 to 1996, expenditures for diabetic lower extremity ulcer patients have been estimated to be on average three times higher than costs among Medicare patients in general ($15 309 vs $5226), accounting for $15 billion in 1995 for the whole US Medicare system [20]. In line with other studies, most of the costs accrued on the inpatient side (73.7%).
Although these cost estimates were based on selected populations using less specific diagnostic data, they provide a useful impression of the enormous economic impact of diabetic foot ulcerations in the health care system.
Hospital-Based Studies
Because hospitalization is the major cost factor in diabetic foot care, a number of studies have investigated this sector in more detail. In the Netherlands, about 20% of all hospitalizations in the diabetic population have been estimated to be related to diabetic foot complications, which was similar to the findings of a previous study from the US [21]. As expected, length of hospital stay (LOS) was the most important cost determinant. Average LOS for diabetic foot problems was 40 days in 1988 in Dutch hospitals, resulting in total costs of about 39 million ECU (US$53 million) (Table 6.3). Amputation was performed in one out of three of these elderly diabetic patients, mainly depending on the presence of osteomyelitis and on ulcer severity. LOS was significantly higher in patients who underwent an amputation (7.5 weeks), but was still five weeks on average even without amputation.
Another more recent study from the Netherlands found an average LOS of 42 days per hospitalization for diabetes-related lower extremity amputations in 1992, which was lower than in the previous study (50 days) [22]. Average hospital costs per amputation were estimated as US$15 330. Costs increased significantly with the age of the patients, a higher level of amputation, and the presence of multiple amputations.
In the US, about 60 000 diabetes-related amputations were performed per year [23]. The direct medical annual costs have been estimated at $300–$500 million. Direct mean hospital charges for primary amputations ranged from $20 000 to $28 000. The average LOS for diabetes-related amputations was shorter in the US (16–40 days) than in the Netherlands. It is noteworthy that among African Americans the average LOS and costs of amputations were substantially higher, which can be explained by a higher prevalence of comorbidities (coronary heart disease, nephropathy) [23].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree