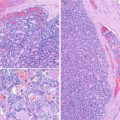
Fig. 4.1
Poorly differentiated thyroid carcinoma (PDTC) . This case of PDTC discloses solid growth pattern, foci of necrosis and invasion of the capsule (a), as well as invasion of one large vessel of the capsule (b). The nested pattern is highlighted by artefactual retraction spaces that some PDTC can present (c)
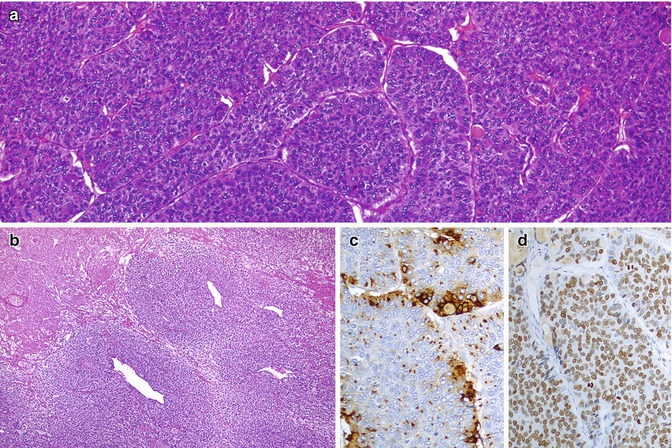
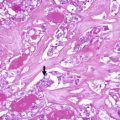
Fig. 4.2
Poorly differentiated thyroid carcinoma (insular carcinoma). This PDTC display a predominant growth pattern in solid nests (insular type) (a). There is tumour necrosis accompanied by preservation of the tumour cells surrounding the vessels resulting in a so-called peritheliomatous appearance (b). Immunohistochemically, PDTC show a characteristic pattern of microfollicular/paranuclear dot-like staining for thyroglobulin (c) and expresses TTF-1 (d), TTF-2, PAX8 and BCL2. Focal expression of p53 is also frequently observed
The case depicted in Fig. 4.1 is a small cell PDTC diagnosed in a 53-year-old female. The nodule had 6 cm in largest dimension. Two years after the diagnosis, the patient developed bone metastases and refractoriness to radioactive iodine treatment.
Small Cell Variant of Medullary Thyroid Carcinoma
MTC is a thyroid tumour histotype that can present multiple aspects [8, 9], mimicking the most frequent follicular cell-derived tumours, as well as some rare entities such as vascular tumours and melanoma. The small cell phenotype of MTC is made by neoplastic C cells and frequently raises problems regarding the distinction from other tumours with small cell phenotype (Fig. 4.3). The immunoexpression of calcitonin is the key diagnostic feature. If calcitonin expression is not detected, one may be facing an atypical form of MTC (see below) or another small cell tumour with or without neuroendocrine features, including paraganglioma and metastatic carcinoma from elsewhere. Calcitonin and TTF-1 expression can be observed also in neuroendocrine carcinomas of the lung metastasizing to the thyroid.
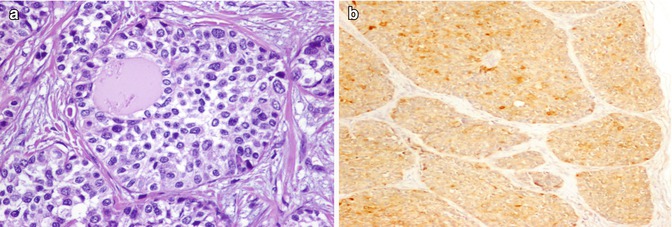

Fig. 4.3
Small cell variant of medullary thyroid carcinoma (MTC) . Small cell variant of MTC disclosing a solid growth pattern around pre-existing benign follicles (a) and expressing calcitonin (b)
The case illustrated here (Fig. 4.3) was from a 44-year-old male with a large thyroid nodule that was diagnosed as a small cell variant of MTC. The patient developed lymph node and lung metastases and died of the disease soon after the initial diagnosis.
Calcitonin-Negative (Atypical) Medullary Thyroid Carcinoma
In the thyroid gland , primary neuroendocrine tumours encompass MTC and, rarely, other tumours such as paragangliomas . MTCs are derived from C cells and express calcitonin and neuroendocrine tumours markers. Some reports have documented thyroid neuroendocrine displaying little, or no expression, of calcitonin that raise difficult diagnostic problems [10, 11]. Figures 4.4 and 4.5 illustrate two cases of C-cell-derived calcitonin-free neuroendocrine carcinoma of the thyroid. These cases presented in a 48-year-old woman and in a 76-year-old man, respectively, with no family history of MTC. In both cases, the tumours were solid and well circumscribed, measuring 28 and 60 mm in largest diameter. Histopathologically, the neoplastic cells formed solid nests surrounded by thin fibrovascular septa with an organoid (“Zellballen”) pattern resembling the aspect of paraganglioma-like MTC . The immunohistochemical findings were similar in both cases. The tumour cells showed reactivity for chromogranin A , synaptophysin , TTF-1, PAX8, cytokeratins (clone AE1/AE3, CK7, CK8 and CK18) and calcitonin gene-related peptide (CGRP) and negativity for calcitonin , CEA, TTF-2 (FOXE1), thyroperoxidase and thyroglobulin. In situ hybridization showed that the neoplastic cells lacked calcitonin and thyroglobulin mRNA expression. Genetic analysis did not disclose any RET mutation. CGRP is a member of the calcitonin family of neuropeptides, which is generated as a consequence of tissue-specific mRNA alternative splicing of the CALCA gene-encoding calcitonin, CGRP and catakalcin (procalcitonin). CGRP is also produced in other organs; therefore, CGPR expression alone does not necessarily indicate the C-cell origin of the tumour cells. However, together with the expression of TTF-1 and PAX8, and despite the loss of calcitonin expression, the positivity for CGRP pointed to the C-cell origin of the two tumours leading to a diagnosis of calcitonin-negative MTC.
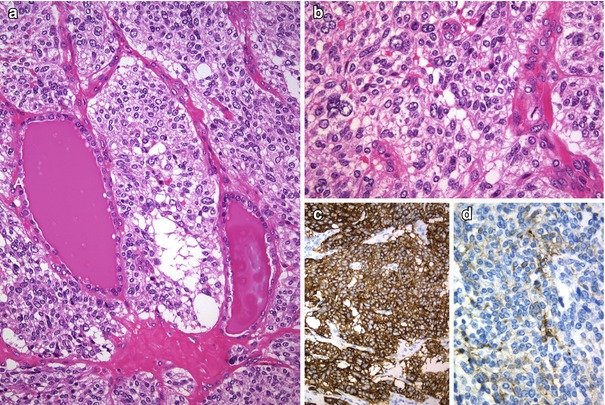
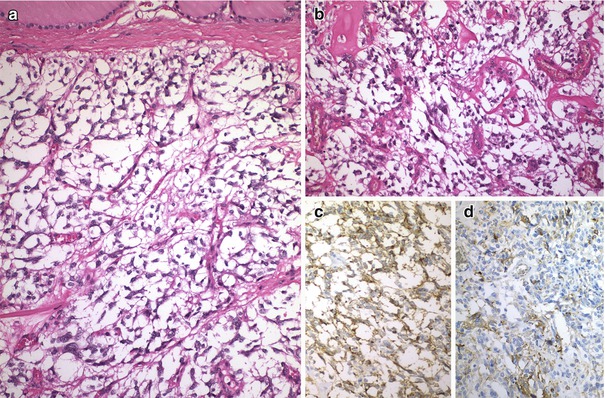

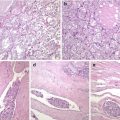
Fig. 4.4
Calcitonin negative (atypical) medullary thyroid carcinoma (MTC) . The tumour was encapsulated and composed of neoplastic cells arranged in middle-sized nests separated by thin-walled blood vessels (a). Tumour cells are round to oval with mild to moderate atypia and have nuclei with slightly coarse chromatin and inconspicuous nucleoli (b). No immunoreaction for thyroglobulin and calcitonin was detected in any tumour cell. Positivity was found for chromogranin A (c), synaptophysin , TTF-1, PAX8, cytokeratins and calcitonin gene-related peptide (CGRP) (d)

Fig. 4.5
Calcitonin negative (atypical) medullary thyroid carcinoma (MTC). This is a well-circumscribed, angioinvasive tumour without extrathyroid extension (a). There were fewer than 1 mitotic figure per 10 high-power fields, and no foci of necrosis (b). The tumour cells were positive for chromogranin A (c), synaptophysin , CD56, TTF-1, PAX8, cytokeratins and CGRP (d) and were negative for calcitonin , CEA and thyroglobulin. The patient remains free of disease 18 months after left lobectomy plus isthmectomy
In the follow-up of patients with MTC, serum calcitonin level is a crucial tumour marker. The lack of calcitonin expression in MTC raises a problem similar to the absence of thyroglobulin expression in differentiated thyroid carcinomas: the marker (calcitonin) may not represent the tumour burden and may not be useful for the follow-up.
Thyroid Lymphoma
Lymphomas of the thyroid, including MALT-type B-cell lymphoma, T-cell lymphoma and follicular lymphoma , disclose also a small cell phenotype but are less frequent than the diffuse large B-cell lymphoma composed of larger lymphoid cells. Lymphomas may involve the thyroid as part of a systemic disease or, rarely, as a primary disease originating in the thyroid (primary lymphoma) [12]. Many patients are elderly women that complain of a rapidly growing mass and compressive symptom, simulating an anaplastic thyroid carcinoma. The histological features of each lymphoma type in the thyroid are similar to those of corresponding lymphomas in other locations. The overall destruction of the thyroid architecture and the prominent lymphoepithelial lesions, together with the coexistence of plasma cell differentiation, are morphological clues that favour the diagnosis of MALT-type B-cell lymphoma against the alternative diagnosis of florid Hashimoto thyroiditis (Figs. 4.6, 4.7 and 4.8). In most cases, the diagnosis of lymphoma involving the thyroid can be made by FNAB and flow cytometry. The case illustrated in Fig. 4.5 is from a 58-year-old female with an enlarged thyroid without nodules and a diagnosis of MALT-type B-cell lymphoma primary of the thyroid. No other foci of lymphoma were found in the patient.
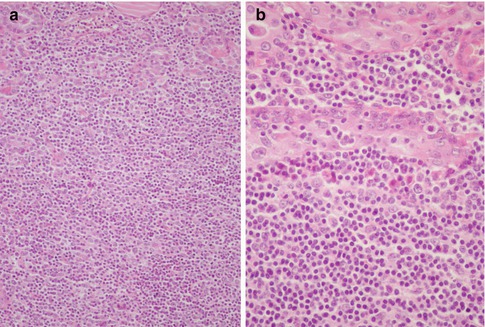
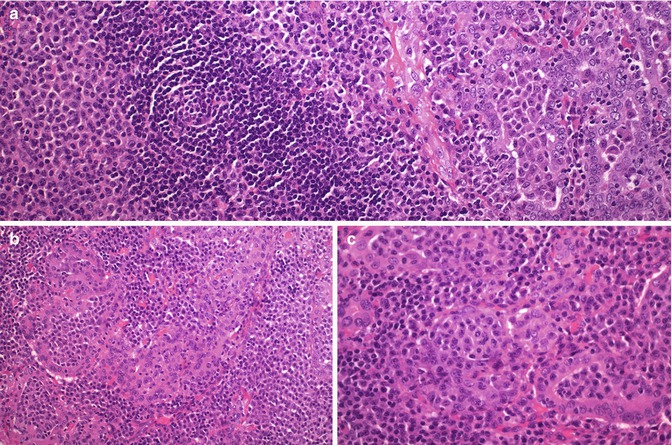

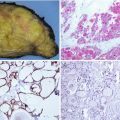
Fig. 4.6
Primary MALT-type B-cell lymphoma of the thyroid. The tumour discloses an extensive effacement of the thyroid architecture due to a diffuse proliferation of small lymphoid cells (a) and lymphoepithelial images that consist in the permeation by neoplastic B-cell lymphocytes of the epithelial cell nests that remain from the original thyroid tissue (b)

Fig. 4.7




Marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) of the thyroid. This case presented in a 73-year-old woman with a history of lymphocytic thyroiditis and rapid enlargement of the thyroid. There was an extensive infiltration of the thyroid parenchyma by sheets of small lymphoid cells, centrocyte-like cells, monocytoid cells and plasma cells, often with interspersed reactive lymphoid follicles (a). Malignant cells tended to infiltrate and expand thyroid follicles forming lymphoepithelial lesions. The plasmacytoid nature of the infiltrate and the packing of follicles by the tumour cells are important diagnostic clues (a–c)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree