Type of cancer
Incidence rate
Common site
Risk factors
5-year survival
Carcinoid
37–44 %
Ileum (44 %)
MEN1a
60–75 %
Adenocarcinoma
33–36 %
Duodenum (55 %), jejunum (30 %), ileum (11 %)
Crohn’s disease, celiac, FAPb
26–39 %
Lymphoma
15–17 %
Ileum (66 %), jejunum (20 %)
Celiac, AIDSc, EBVd
25–40 %
GISTe
7–9 %
Jejunum
40 %
Metastasis
Melanoma (42 %), breast (16 %), lung (12 %)
Melanoma in jejunum and ileum
Primary cancer
Solitary lesion with good prognosis
Etiology and Pathogenesis
The small bowel comprises the majority of the gastrointestinal tract but has a very low cancer incidence. The low risk of developing SBA is likely due to a number of factors including rapid transit of contents, decreased amount of exposure of carcinogenic substances, high amount of lymphoid tissue, high amount of IgA secretion, rapid turnover of cells, and the alkaline nature of the small bowel contents [3, 4]. The most common modifiable risk factors appear to be cigarette smoking and alcohol consumption when compared to nontobacco users and nondrinkers. There are some underlying conditions that may increase the risk of small bowel cancer. Patients with celiac disease and Crohn’s disease have an increased risk of both adenocarcinoma and lymphoma. Familial adenomatous polyposis (FAP) patients will have risk of duodenal and periampullary adenocarcinoma. Patients with hereditary non-polyposis colorectal cancer (HNPCC) develop adenocarcinomas of the small intestine at younger ages than the general population.
The low incidence rate and lack of screening modalities have led to a delay in diagnosis and more advanced disease at the time of presentation. The primary presenting symptoms are nonspecific and include abdominal pain, gastrointestinal bleeding, jaundice, weight loss, abdominal distention, nausea, vomiting, and anemia. The nonspecificity of the symptoms leads to a delay in diagnosis and treatment, ranging from 2 months to 12 months [5]. For this reason, 30–50 % of small intestine cancers are associated with nodal or distal metastases at the time of presentation. The median survival time of patients diagnosed of small bowel cancer is 13–20 months with a 5-year OS ranging from 0 to 28 % for adenocarcinoma, 14–30 % for lymphoma, and 60 % for carcinoid [5]. Several poor prognostic factors are age >75 years, duodenal site, TNM staging, nodal involvement, and positive surgical margins [6].
Diagnostic Tools
Historically, the diagnostic difficulty of evaluating the small bowel adequately leads to delays in diagnosis and risk for more advanced disease. This has encouraged new innovation in the evaluation of small intestine cancers. The most appropriate diagnostic modality includes a combination of noninvasive imaging and endoscopy with biopsy capability. Barium enteroclysis was, historically, the most common imaging offered to evaluate the small bowel. This was time consuming, poorly tolerated by the patient, and still not able to accurately depict mural and extramural extent of disease. Improvements in computed tomography (CT) and magnetic resonance imaging (MRI) modalities and improved small bowel enteroscopy with capsule endoscopy or double-balloon endoscopy add to the surgeon’s armamentarium.
Computed topography and MRI enteroclysis are other modalities of imaging the small bowel. These radiographic modalities require the patient to fast the day prior to the study. The patient is asked to consume an oral agent to allow for optimal small intestine distention prior to the study. The diagnostic yield for both CT and MRI is similar. The advantages of CT scanning include more hospitals are equipped with CT and the patient procedural time is much less. MR imaging avoids radiation and bowel distention can be monitored dynamically to achieve the best imaging.
Various endoscopic techniques are employed for evaluating small bowel tumors. Traditional endoscopy is the gold standard for evaluating the gastrointestinal tract because of its imaging, diagnostic, and potential therapeutic capabilities. Lesions in the small bowel proximal to the ligament of Treitz are usually viewed and biopsied by conventional endoscopy. Push endoscopy is another endoscopic technique for evaluating the duodenum and 50–70 cm past the ligament of Treitz. More distal lesions are now visualized with the evolution of capsule endoscopy (CE) and double-balloon enteroscopy (DBE). With CE, the patient swallows the capsule and a signal is sent to a recording device that the patient wears on their belt. DBE, also known as push-and-pull enteroscopy, enables visualization of the entire small bowel.
Capsule endoscopy and DBE have opened new avenues for imaging small intestine cancers and as an alternative diagnostic option. The CE is a highly effective way to image the entire length of the small bowel but is limited to mucosal lesions and does not have biopsy capability. Double-balloon endoscopy is suggested if a suspicious lesion is identified on CE to obtain tissue diagnosis. The disadvantage of DBE is that it requires specialized instruments and a gastroenterologist who is trained in performing the procedure. Kopacova et al. reviewed a series of DBE of 303 procedures in 179 patients in which 74 small bowel tumors were discovered. In this series CE preceded DBE in 21 patients. They were able to detect a suspicious lesion in 20 cases (one tumor missed by CE) [7].
Intestinal Neuroendocrine Tumors (Carcinoid Tumor)
The incidence of intestinal neuroendocrine tumors (iNETs), also known as carcinoid tumors, has increased significantly over the past three decades. This section will focus on the small intestine manifestations of neuroendocrine tumors. Discussions of lung, appendiceal, pancreatic, rectal, and stomach tumors are located in other chapters of the book (Chap. 24, Pancreatic Neuroendocrine Tumors (PNETs); Chap. 26, Carcinoid Tumors). The reason for the increase in diagnosis is poorly understood but may be related to incidental findings secondary to better imaging. Neuroendocrine tumors arise from the enterochromaffin cells in the crypts of Lieberkuhn and produce vasoactive peptides, mainly serotonin. These tumors cause a significant desmoplastic reaction, causing obstructive symptoms or bowel ischemia if the mesenteric vessels are involved (Fig. 10.1; Table 10.1). Surgical resection and lymphadenectomy is the mainstay of curative treatment. Somatostatin and somatostatin analogs are key in the treatment of symptomatic or metastatic disease, but other chemotherapy agents have evolved. Overall prognosis of regional intestinal neuroendocrine tumors is 60–75 % over 5 years [1].
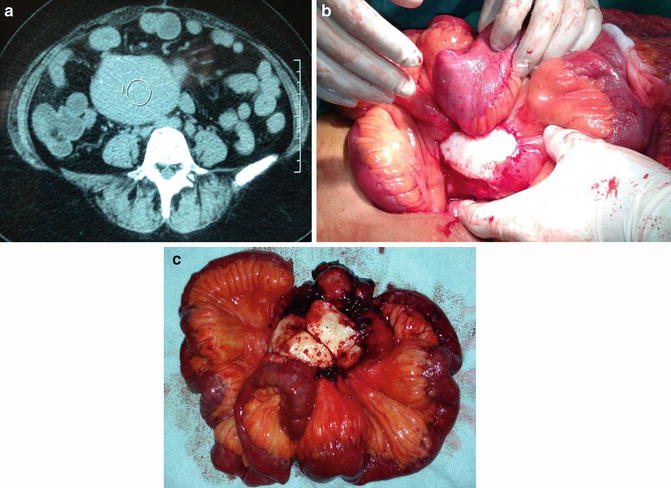

Fig. 10.1
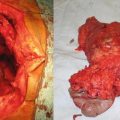
Small bowel carcinoid: noncontrast CT shows a large intra-abdominal mesenteric-based mass (a). Intraoperative the lesion had significant desmoplastic involvement of the small bowel mesentery (b, c). (Courtesy of Gazi B. Zibari, MD, FACS)
Neuroendocrine tumors often have an indolent growth pattern compared to similar carcinomas, and early detection can lead to curative treatment. Most small intestine tumors are located within 60 cm of the ileocecal valve but may be present anywhere in the gastrointestinal tract. Conventional CT scans with oral and IV contrast are usually sufficient to detect a primary lesion. However, carcinoids are characterized by a high potential to metastasize to other areas of the intestine or the small bowel mesentery. Synchronous tumor may be found in up to 40 % of patients at the time of laparotomy for the primary lesion. Midgut neuroendocrine tumors retain their expression of somatostatin receptors which bind octreotide. Somatostatin receptor scintigraphy with indium-111 pentetreotide has an 86–95 % sensitivity for detecting extrahepatic foci or distant metastases [8, 9]. Scintigraphy is indicated in patients for staging purposes and determination of receptor status. Somatostatin receptor (sst) expression, particularly sst2 receptor, has been correlated with better response to analog therapy and confers a better prognosis[8, 9]. Small bowel imaging with endoscopy and chest CT scan may be helpful preoperatively to further plan surgical resection and metastasis.
Patients with liver or lung metastasis are prone to the development of the carcinoid syndrome, a constellation of symptoms which includes flushing, diarrhea, and valvular heart disease. The carcinoid syndrome is caused by secretion of serotonin and other vasoactive substances into the systemic circulation. Serum chromogranin A is produced by all neuroendocrine tumors and has 86 % specificity and 68 % sensitivity. Twenty-four-hour urine collection for 5-hydroxyindolacetic acid (5-HIAA), a common metabolite of serotonin, can be used in the diagnosis and postoperative follow-up. Patients with carcinoid syndrome should have a 2D echocardiogram prior to any surgical procedure to evaluate cardiac function and valvular fibrosis [10].
Surgery is the cornerstone in the treatment of neuroendocrine tumors, but the location of the tumor is critical in surgical planning. Jejunal and ileal regional disease is commonly treated with bowel resection and lymphadenectomy. The goal of the lymph node resection is to have >12 nodes present for evaluation. Cholecystectomy is indicated during the initial surgical resection due to possible future long-term somatostatin analogue therapy and an increased risk of biliary symptoms. Duodenal tumors <10 mm and limited to the submucosal layer may undergo endoscopic submucosal dissection with low risk of perforation or bleeding [11]. Larger tumors (>10 mm) of the duodenum or involvement of the ampulla may require pancreaticoduodenectomy to obtain surgical margins (Fig. 10.2). These patients should be followed every 3–12 months with repeat 24 h urine 5-HIAA and chromogranin A tests with serial abdominal and pelvic CT scan [10].
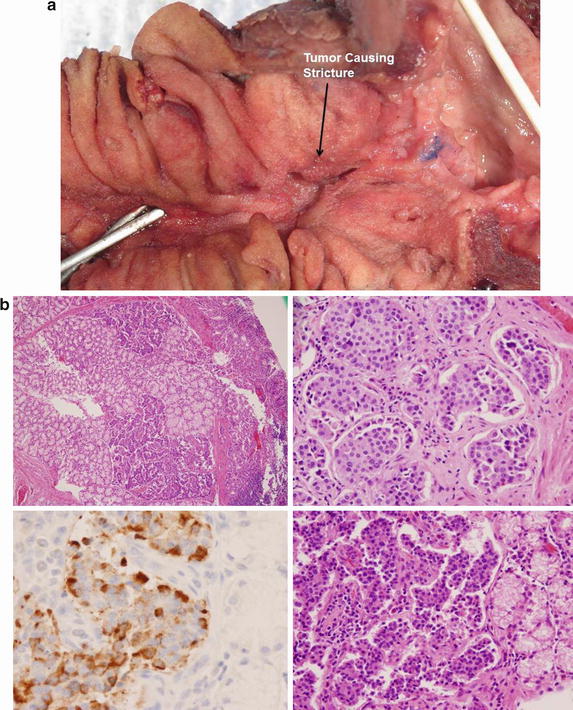

Fig. 10.2
Neuroendocrine tumor of the small bowel: a 61-year-old man presented with nausea and vomiting; an upper endoscopy demonstrated a tight stricture at the second portion of the duodenum. The CT scan of the abdomen demonstrated no mass or abnormality in the head of the pancreas or biliary system. He underwent a pancreaticoduodenectomy and tight stricture that was caused by a neuroendocrine tumor of the duodenum was found (a). H&E stain demonstrates “small blue cell tumors” that have scant, pink granular neoplasm, and round to oval stippled nucleus (b). The tumor stained positively for gastrin. CD31 immunostain highlights scattered vessels. Ki68 proliferation index was <2 % and was low grade, and the final stage was pT1pN1. (Courtesy of Quyen D. Chu, MD, MBA, FACS)
More aggressive surgical approaches in patients with metastatic disease have emerged over the past decade. In concordance with the most recent NCCN guidelines, cytoreductive surgery may be indicated in patients with local symptoms; localized tumor metastasis or >90 % of tumor burden can be resected or ablated [12]. The United Kingdom and Ireland Neuroendocrine Tumor Society (UKI NETS) study conducted evaluated 360 patients with midgut neuroendocrine tumors with hepatic metastasis and found resection of primary lesion and metastasis had a median survival of 11.26 years compared to 5.50 years in patients without surgical intervention [13]. Hepatic radiofrequency ablation or arterial embolization can be considered for multifocal disease that is not amenable to surgical resection. Complete or partial response for symptomatic improvement, tumor markers, and imaging occurred in 70–100 %, 50–90 %, and 30–50 % of patients, respectively [14].
Medical treatment options for metastatic unresectable iNETs have expanded in recent years but are not considered curative. The goal of medical therapy is antisecretory effects and antiproliferation of the existing tumor. Of particular importance has been the development of somatostatin-analogue therapies. Somatostatin analogues were originally introduced for palliation of the carcinoid syndrome; however, recent clinical trials have demonstrated that they can exert an inhibitory effect on tumor growth. Common systemic chemotherapy regimens have been modeled after the PROMID (Placebo Controlled, Double-blinded, Prospective, Randomized Study of the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine MIDgut tumors) study with octreotide long-acting release (LAR) and the Controlled Study of Lanreotide Antiproliferative Response in NET (CLARINET) trial. Current recommendations suggest first-line agents of somatostatin analogues (SSA) of octreotide or lanreotide every 4 weeks for patients with low volume stable disease. If symptoms occur or worsen, the dose of SSA can be increased and alpha interferon or an mTOR inhibitor, everolimus, may be added. Nonresponders to these agents may proceed to systemic chemotherapy with 5-flurouracil, capecitabine, and oxaliplatin. Third-line therapy includes the antiangiogenic targeting agents sunitinib or bevacizumab, which have shown evidence of improving progression-free survival [12].
Overall survival (OS) and progression-free survival (PFS) have improved in the past three decades with better surgical technique, understanding of tumor biology, and development of SSAs. The overall 5-year survival of an iNET is 60–75 %. Progression-free survival at 5 and 10 years for AJCC/UICC has been shown as 100 % stage I (5 years), 100 % stage II (5 years), 86 and 63 % stage III, and 64 % and 19 % for stage IV disease [15]. Unfavorable prognostic factors impacting survival include high mitotic rate, high Ki67, distant metastasis, and advanced age. Octreotide scintigraphy avidity suggests that even with hepatic metastasis, avid uptake of the nuclear marker has a better prognosis than those that had low or no uptake.
Adenocarcinoma
Small bowel adenocarcinoma (SBA) is the second most common type of cancer in the small intestine. This cancer has a tendency to present late in the course with 50 % of patients having advanced disease at the time of presentation. The adenocarcinoma sequence similar to colon cancer is thought to play a role but has not been fully elucidated yet. Patients may be at an increased risk due to a higher consumption of animal fat and protein in the Western diet or diagnosed with HNPCC, FAP, or Crohn’s disease. The duodenum is the most common site of involvement (55 %) followed by jejunum (18 %) and ileum (13 %) (Table 10.1). Ileal adenocarcinoma is increased in Crohn’s patients. Patients with periampullary tumors present with nausea, vomiting, and obstructive jaundice. Ampullary tumors are biologically and morphologically distinct and have a more favorable prognosis compared to pancreatic and bile duct cancers. Tumors in the jejunum and ileum commonly present with obstruction, weight loss, and frequently have lymph node involvement.
Surgery is the mainstay for curative treatment of patients with SBA (Fig. 10.3); unfortunately, many patients (32 %) have evidence of metastasis at the time of resection. Typically, adenocarcinomas are staged according to the AJCC TMN staging system in Table 10.2 based on tumor invasion, node status, and metastasis. Lymph node status and distant metastasis implicates a poorer prognosis and is associated with a decreased 5-year relative survival rate with localized disease 80.5 %, regional with lymph node involvement 68.5 %, and distant metastasis 41.5 %. The number of lymph nodes required for R0 resection was reviewed by Gibbs in 2004; the current consensus of obtaining ≥10 lymph nodes is adequate without adding increase morbidity [16]. Factors that may predict a worse outcome are male sex, patients older than 55, tumor size, and poorly differentiated tumor or with positive margins. In our review of 13 published papers from 2000 to 2011 patients with SBA, the efficacy of adjuvant treatment after curative intent surgery did not improve overall survival. Table 10.3 displays the characteristics of the populations studied listed by the author and year [17–28]. In these papers curative surgery was either bowel resection including lymphadenectomy or pancreaticoduodenectomy (Whipple procedure) with negative margins. Distant metastasis after surgical resection is the most common pattern of relapse and adequate adjuvant therapy is needed. Table 10.4 shows 5-year OS and median survival time for patients with SBA.
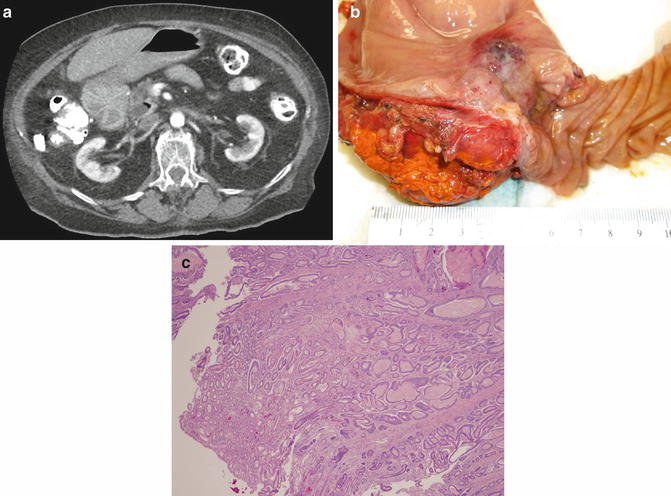

Fig. 10.3
Small bowel adenocarcinoma. A 65-year-old woman presented with a duodenal obstructing mass. On CT there is evidence of a tumor partially occluding the duodenal lumen (a). On gross pathologic inspection following a pancreaticoduodenectomy, the mass is felt to arise from either the duodenal or the ampulla (b). Final pathology revealed both duodenal and ampullary dysplasia with associated invasive adenocarcinoma. Although pathology favored ampullary adenocarcinoma, they could not definitely rule out a duodenal source. The photomicrograph of a separate patient demonstrates small bowel adenocarcinoma arising from a background of dysplasia (c) (Courtesy of John F. Gibbs and Joyce Paterson)
Table 10.2
American Joint Committee on Cancer (AJCC) TNM staging for small intestine cancer (7th edition)
Primary tumor (T) | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ |
T1a | Tumor invades lamina propria |
T1b | Tumor invades submucosa* |
T2 | Tumor invades muscularis propria |
T3 | Tumor invades through muscularis propria into the subserosa or into the nonperitonealized perimuscular tissue (mesentery or retroperitoneum) with extension 2 cm or less* |
T4 | Tumor perforates the visceral peritoneum or directly invades other organs or structures (include other loops of small intestine, mesentery, or retroperitoneum >2 cm, and abdominal wall by way of serosa; for duodenum only, invasion of pancreas or bile duct) |
*The nonperitonealized perimuscular tissue is, for jejunum and ileum, part of the mesentery and, for duodenum in areas where serosa is lacking, part of the interface with the pancreas | |
Regional lymph nodes (N) | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Metastases in 1–3 regional lymph nodes |
N2 | Metastases in ≥4 regional lymph nodes |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis |
Anatomic stage/prognostic groups | |||
|---|---|---|---|
Group | T | N | M |
Stage 0 | Tis | N0 | M0 |
Stage I | T1-T2 | N0 | M0 |
Stage IIA | T3 | N0 | M0 |
Stage IIB | T4 | N0 | M0 |
Stage IIIA | Any T | N1 | M0 |
Stage IIIB | Any T | N2 | M0 |
Stage IV | Any T | Any N | M1 |
Table 10.3
Characteristics of studies reviewed for small bowel adenocarcinoma
Author, year | N | Mean age range | Male % | Stage breakdown | ||||
|---|---|---|---|---|---|---|---|---|
I | II | III | IV | Grade definition | ||||
Ojha, A. 2000 | 33 | 57.5 | 64 | I–III 24 | IV 9 | n/a | ||
North, J. 2000 | 68 | 15–89 | 70 | n/a | n/a | |||
Fishman, P. 2006 | 113 | 58.2 | 59 | 30 | 41 | 32 | Poor vs. well | |
Agarwal, S. 2007 | 64 | 17–87 | 57 | 1 | 14 | 21 | n/a | |
Kelsey, C. 2007 | 32 | 32–77 | 72 | 3 | 18 | 2 | 1 | Poor vs. mod and well |
Chaiyasate, K. 2008
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||||||