Fig. 5.1
Left: Pattern of absorption of a primitive diet; Right: The effect of a highly absorbable modern diet (Personal file)
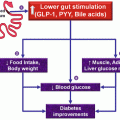
Obviously, the proximal gut would be over-stimulated in this scenario, while the distal gut under-stimulated, as nutritive absorption occurs more proximally than what is “naturally” expected: an imbalance. Indeed, in obese and diabetic patients present high GIP (Vilsboll et al. 2003) (mainly a proximal gut product). If they loose weight on a diet, GIP falls (Deschamps et al. 1980). GIP is obesogenic and insulinotropic (contributing to hyperinsulinemia) (Miyawaki et al. 2002), and the therapeutic blockage of GIP has been shown to be beneficial in MS patients (Irwin and Flatt 2009; Montgomery et al. 2010).
The opposite is also very clear. The therapeutic addition of the distal gut products or agonists (GLP-1, PYY, oxyntomodulin) (Bhavsar et al. 2013; Batterham et al. 2002, 2003; Neary et al. 2005; Pocai 2012) is beneficial in MS patients.
Drugs and hormones that interfere in this balance, by enhancing the distal or blocking the proximal gut activity, help MS patients. Surgeries reproduce this observation. The more you shift food from proximal to distal, better the results in both, obesity and MS.
There is an imbalance in gut activity, and the introduction of easily absorbed food can completely justify that. Epidemiology, physiology, drugs, and surgeries point at the same direction.
5.1.3 A Contribution from Biology and Anthropology
Nature, through the means of evolution, adjusts permeability and extension of GI tracts of mammals, according to the quality of diet, utilizing natural selection.
Mammals do not digest fiber directly, but uses bacterial fermentation to obtain some nutrients (mostly short chain fatty acids). Some mammals do it in the forestomach (like ruminants); some do it in the colon (like horses, apes, and humans). The characteristics of the GI tract vary among species, mostly guided by the characteristics of diet. As a general rule, high-fiber, low-protein diet implies large and long GI tracts, able to process this type of food and also get nutrients from fermentation.
Small herbivores, such as rodents, that are not big enough for containing the necessary GI tract to this diet, sometimes use coprophagy (stool eating) as ways to process food twice, as if they had twice as much gut (Stevens and Hume 1995).
Facing richer food, like the carnivores do, evolution has been selecting simpler and shorter GI tracts (Fig. 5.2), putting distal bowel nutrient detectors closer, since more nutrients are absorbed in shorter segments.
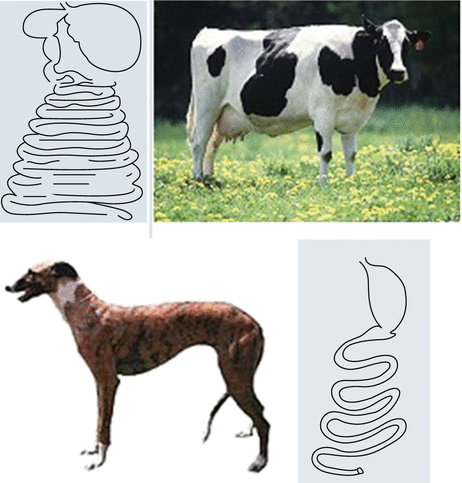

Fig. 5.2
High calorie-density diet (as in carnivores like a dog) is associated with shorter and simpler gut, and with a smaller gut/body ratio, as a rule among all mammals. Animals whose diet is fiber-rich and calorie-poor (as in herbivores like a cow) present a high gut/body ratio (Gastroenterocorporeal ratio) (Personal file)
Primate evolution follows this rule. Australopithecus afarensis, an early hominid specimen that lived about 2.5 million years ago, was mainly an herbivore. Plant food became scarce, contributing to the extinction of the Australopithecine, while other groups of hominids started adding more caloric animal food, being able to eat less volume of more digestible food. It is known that in this transition from Australopithecus to Homo genus, the amount of bowel was reduced as expected (Schmid 1983; Aiello and Wheeler 1995) (Fig. 5.3).
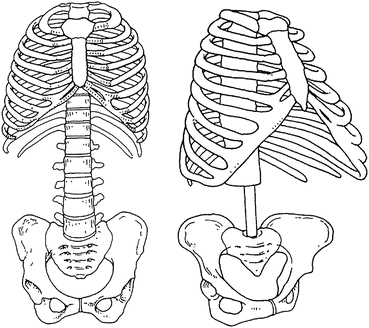

Fig. 5.3
The human abdominal cavity became smaller (left), when compared to primitive Australopithecus afarensis (right) (Modified from Schmid 1983)
In a quite long period of time we moved from being gatherers to hunter-gatherers. But, in a biological short period, agriculture came and gathering was transformed in harvesting. Hunting became slaughtering. After that, in a few centuries, even greater changes happened in human diet with refining and with industrialization. Hydration, which is obtained with water by all other animals, became a nutritive process with the development of juices and soft drinks.
Our diet became more concentrated in calories and even freer of non-digestible particles, which may lead us to think that our gastrointestinal (GI) tract is now unfit for this diet, especially if in large amounts. From this point of view, our GI tract was developed under scarcity and a poorer diet, which we don’t face anymore, leading to a condition of “proximal-distal gut imbalance.”
Evolution is doing its routine job: Selection. Some people, in face of food abundance, are becoming obese, sick, and dying earlier. So, as expected, smaller abdomens continue to be selected. Epidemiological observations confirm this rationale. Waist circumference has been pointed as a risk factor.
Sleeve gastrectomy (SG) copies natural (evolutionary) movements. It reduces gastric capacity, and smaller volumes will generate functional restriction signals earlier. The resection of the gastric fundus reduces A cell population, which produces ghrelin (a pro-appetite hormone), and parietal cell population (Langer et al. 2005; Muccioli et al. 2002).
The low gastric pH reduces the bacterial population in the food, which is low in the small bowel and becomes large again in the colon, due to the necessity of bacterial participation in the process of fiber fermentation. Simultaneously, the acid has a role in digestion, especially by activating pepsinogen and by participating in protein digestion. The less contaminated and more caloric modern diet requires a smaller amount of acid. Indeed, modern populations suffer a lot more from acid excess than from the lack of it. A vertical gastrectomy reduces very much the capacity of acid production, with no reports of problems related to this decrease.
A SG keeps outer stomach functions quite well. Innervation through the lesser curvature is intact. Pylorus works, providing a gradual and controlled emptying, with adjustment of osmolarity, preventing dumping and adjusting the progression of particles with different size. Bacterial population will be diminished by the action of acid, produced by the remaining parietal cells. Gastric secretions also stimulate duodenal and pancreatic secretions, while initiating protein digestion. There are no reports of lack of vitamin B12, which could be a problem if the amount of intrinsic factor (produced by gastric mucosa and fundamental to absorption of vitamin B12 in the ileum) were insufficient.
In summary, a vertical gastrectomy produces a decrease in gastric size; gastric function is quite well preserved (Gumbs et al. 2007) (Fig. 5.4).
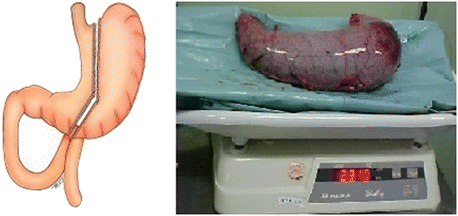

Fig. 5.4
Pattern of resection in a vertical gastrectomy (left). Gastric specimen (right), full with water (Personal file)
It is an efficient procedure for weight loss (Langer et al. 2005). Differently from RYGB, in which the fall in ghrelin is controversial (Stoeckli et al. 2004; Holdstock et al. 2003; Le Roux et al. 2003), a vertical (sleeve) gastrectomy produces a significant decrease of ghrelin (Adkins and Davies 1987).
In summary, based on what is known now, it seems that a vertical gastrectomy is the maximum procedure that can be performed in the stomach for the treatment of obesity, without major disturbance in gastric functions.
5.2 The Term “Bariatric”
To move forward, we have to recognize that “bariatric” became an old and inadequate name. Bariatric refers to weight. Weight itself is not the problem, as heavy people may not be obese. Obesity and metabolic syndrome (MS) are the problem, and they may happen sometimes with not too much weight. They are metabolic problems. By calling the surgery “bariatric,” it means we aim at the weight; surgical indication is guided by the weight, and the promise is a normal weight. Bariatric surgery uses mechanical restriction and malabsorption. Both are diseases, classified in the International Classification of Diseases (K.90 responds to Malabsorption) and they are not physiological at all.
If we call it “metabolic,” a lot changes. We aim at adjusting a metabolic response to food ingestion. The surgical indication is metabolism driven. The surgical main goal and the promise is not the weight, even because the weight does not depend on the surgery only. The goal and the promise is a metabolic change, which will help the patients in obtaining better rewards for their efforts in maintaining good health, good weight, normal glycemia, normal blood pressure, and normal blood lipids.
5.2.1 Pure Metabolic Surgery
Bariatric surgery with its tools, mechanical restriction, and malabsorption has produced unexpected beneficial metabolic results. The discovery that these results were a product of an array of different metabolic events, later created the term “bariatric and metabolic” surgery, in which the procedures are the same old ones, but with the recognition that other new mechanisms are also at work.
Between 2008 and 2011 sleeve gastrectomy (SG) was the only surgical procedure to treat obesity with an increase in the absolute number of procedures: it moved from 18,098 to 94,689: a +523 % difference, while all the others diminished in absolute numbers (Buchwald and Oien 2013). Probably it is because a good SG is the first recognized procedure that fits quite well in the concept of pure metabolic surgery (PMS) (a bad SG may be mechanically restrictive). Ideally, PMS does not bring new diseases on purpose to treat old ones.
5.3 Our Surgical Strategy
It is important to recognize that obesity grade I (BMI between 30 and 35 kg/m2) has negative effects on health and quality of life and some patients, even under well conducted clinical treatments, cannot achieve weight loss. They remain in this condition in which clinicians cannot treat them and surgeons do not offer an alternative. Even a little overweight patients, but already suffering from severe comorbidities, like orthopedic incapacitating conditions or type 2 diabetes, might eventually be helped by surgeons, especially if we can adjust procedures to patients. However, up to the present day, just patients over 35 kg/m2 of BMI are candidates to procedures aiming at weight loss.
A fast and constant change is observed in these regulatory matters and with little doubt, they will be soon modified, becoming more flexible and having not just the weight as a primary goal but also other metabolic conditions, especially diabetes.
In the opposite side are extreme obese patients. It is not easily acceptable that they might be treated the same manner as a patient with a 36 kg/m2 BMI.
Sleeve gastrectomy has been proposed as a first step in the treatment of superobese, leaving the duodenal switch or biliopancreatic diversion (BPD) to be performed later (with lower risks) (ASBMS 2012). As the sleeve gastrectomy is a procedure with a very low potential to cause long-term problems, we think it can be used also in the other side of the line, to obese patients who are not heavy enough, nor sick enough to fulfill the traditional conditions to surgical indication. Patients that have conditions to help with exercise and are in the search for a friendly procedure may benefit from a simple sleeve gastrectomy.
These procedures may transform failing clinical treatments in successful ones, aborting progress of obesity and preventing some patients to further need more aggressive therapies.
In some patients, due to severe obesity, severe associated comorbidities, impossibility to exercise, and other conditions where the complete success of bariatric interventions is less probable, it is interesting to have a more potent procedure. We developed transit bipartition (TB) (Santoro et al. 2003). We developed transit bipartition (TB) as a meabolic complement to the sleeve gastrectomy
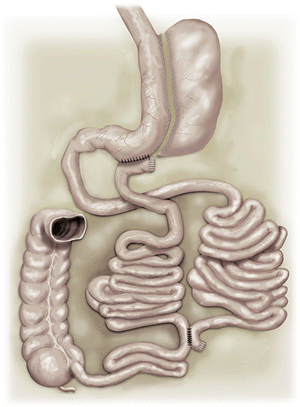
TB is a new concept that we introduced in 2003 (Santoro et al. 2003) to avoid duodenal–jejunal exclusion, because we recognize the importance of the nutritional and endocrine functions of this segment, the adverse consequences of exclusion (nutrient deficiencies, mucosal atrophy, bacterial proliferation, and lack of endoscopic access). TB is a surgical strategy to further reduce the exposition of nutrients to proximal intestinal mucosa without, however, producing exclusions. It consists in taking the proximal ileum to be anastomosed to the stomach leaving the pylorus open, as much as the duodenum, creating a division in the transit.
When compared to the traditional duodenal “switch,” performed in some BPDs, bipartition is easier, avoids anastomoses with the proximal duodenum (a hard task in obese patients), and, additionally, leaves an open duodenum (Fig. 5.5).
5.4 Sleeve Gastrectomy and Transit Bipartition
Evidence from several studies (Haber et al. 1977; Jenkins et al. 1980; Wisén and Johansson 1992; Ranganath et al. 1996; Lugari et al. 2002; Lam and Kieffer 2002; Bojanowska 2005) suggested that an excessive proximal absorption due to dietary interventions could be the cause for enterohormonal disorders. In 1997, Naslund et al. showed that the jejunoileal bypass (JIB), an old bariatric procedure that sends nutrients through a shortcut to the ileum (maintaining the duodenum inflow but excluding most of the jejunum and ileum), caused a long-lasting enhancement in GLP-1 secretion (Naslund et al. 1997). Consequently, it was demonstrated that GLP-1 deficient patients were indeed capable of secreting GLP-1 if distal nutritive stimuli were sufficient. Additionally, it became clear that JIB worked by promoting intentional malabsorption and through unpredicted metabolic ways. Looking back to 1980, this discovery could be explained by the observation documented by Organ et al. (1980), that JIB induced a fast remission of diabetes, before the occurrence of significant weight loss.
In 1998, the world was intrigued by an article (Hickey et al. 1998), that investigated whether type 2 diabetes mellitus (T2DM) could be a disease of the foregut. The nonrestrictive and non-malabsorptive effects of bariatric surgery became a topic of interest.
Based on these elements, we supposed that the mentioned dietetic modifications that intensify proximal absorption indeed diminish distal absorptive work, causing signaling disorders (Santoro 2003, 2008a). A surgical strategy (Santoro 2006, 2008b) was proposed to counterbalance the digestive tract signaling aiming at enterohormonal correction. It was the first strategy originally developed to maximally avoid restriction and malabsorption, instead of inducing them for therapeutic purposes.
This strategy included different procedures capable to cause metabolic interventions. Among them, we observed that sleeve gastrectomy and transit bipartition (SG + TB) were highly effective.
Patients with classic indication for bariatric surgery, that understand and accept that their surgery will basically rely on its metabolic components and not on malabsorption, are offered SG + TB. From June, 2004, until January, 2011, 1020 patients underwent SG + TB (Santoro et al. 2012).
At the time of surgery, patients had body mass indexes (BMIs) ranging from 33 to 72 kg/m2 (average 42.2 kg/m2). Diagnosed comorbidities included orthopedic problems, including joint pain, essential hypertension, diabetes, dyslipidemia, and respiratory problems including sleep apnea. Other frequently occurring preoperative conditions included a high prevalence of abnormally high levels of hepatic enzymes and hepatic steatosis, cholelithiasis, hyperparathyroidism, low blood thiamine, insufficient levels of 25 hydroxyvitamin D, menorrhagia in association with anemia, polycystic ovary syndrome, depression, and anxiety disorders.
5.4.1 Operative Technique
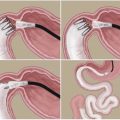
The procedure combines a typical SG with a TB; this creates a shortcut to the ileum while maintaining access to the duodenum (Fig. 5.5). The procedure may be performed in a conventional open method, laparoscopically, or, alternatively, with mixed access, where the SG is performed through laparoscopy, and a mini-laparotomy is formed by the union of two trocar incisions. This approach provides adequate access for the retrieval of the gastric specimen and composes the enteric part of the surgery. Here, we describe the laparoscopic method.
Pneumoperitoneum is obtained using a Veress needle. Six trocars are positioned, including three 12-mm trocars (one in the midline 3–5 cm above the umbilicus and two others in the upper left and right quadrant) and three 5-mm trocars (one in the epigastrium for the liver retractor and two at each lateral flank).
The omental bursa is opened, and the greater omentum is sectioned with a sealer and divider device (Ultracision® or Ligasure®). The greater curvature is freed from 2 cm proximal to the pylorus up to the angle of His, including the left arm of the hiatal crura. If a hiatal hernia is present, then a hiatoplasty is performed.
A typical sleeve gastrectomy (Baltasar et al. 2005) is performed with a laparoscopic linear cutting stapler starting at the gastric greater curvature, at a point located 4–5 cm from the pylorus, up to 0.5 cm from the angle of His. A 36 French bougie is passed to the stomach, to guarantee that the remnant gastric tube, which is positioned by the lesser curvature, has an internal lumen 3 cm wide. A seromuscular running suture is sometimes used to cover the stapling line to reduce bleeding.
After the SG, the ileocecal transition is located. A single stitch is used to mark the point at the ileum 80 cm from the ileocecal valve. The point at 260 cm is then located, and a perforation is made with the cautery to allow the insertion of one arm of the linear stapler into the ileal lumen. Another hole is created in the stomach antrum at the end of the stapling line, by applying the cautery against the bougie’s protuberance. The other arm of the stapler is inserted in the stomach from the patient’s left to the right, toward the pylorus, to create a 3- to 4-cm wide latero-lateral gastroileal anastomosis in an antecolic position. A 3-0 absorbable extra mucosal running suture closes the residual defect.
In the following sequence, the small bowel cranial to the gastroileal anastomosis is laterally widely anastomosed to the ileum, at 80 to 120 cm from ileocecal valve (previously marked), in a lateral–lateral mode. A laparoscopic linear stapler with a 45-mm white cartridge is used for the anastomosis. A nonabsorbable running suture closes the mesenteric borders to prevent internal hernias. Today we use a 120 cm “common channel” to avoid worsening the odor of stools. At the end of the procedure, the segment between both anastomoses is interrupted with stapling and cutting. A closed suction drain, lying along the sleeve gastrectomy staple line, is exteriorized through the lower left port incision. The other laparoscopic incisions are closed.
Patients fast in the first postoperative day (POD), and liquid fractioned meals are offered for the subsequent 12 days. Then, soft solid meals are allowed, in a slow progression toward normal food. Patients are instructed to begin meals with a portion of varied salad, enriched with protein (tuna, salmon, or chicken). Avoidance of refined sugar is advised. The patients are also advised to enroll in a physical activity program, with increasing intensity as weight loss occur. Multivitamins and pantoprazol are prescribed in the first 2 months and maintained for longer if necessary.
5.4.2 Follow-up
Patients are instructed to return after 10 days, 1 month, 3 months, 6 months, one year after the procedure and then annually, bringing blood tests, and at least one abdominal sonography performed around 1 year of surgery. Detected gallstones are surgically treated, frequently simultaneously to plastic surgery. Weight is actually measured and not self-reported. Unfortunately, around 40 % of patients return just in the early postoperative period, and not any more.
Data are collected online using an especially developed software; means, graphics, and standard deviations are immediately updated. Remission of T2DM is defined by HbA1c <6.5 % without oral hypoglycemic drugs or insulin, while improvement is defined as a reduction of at least 25 % in the fasting plasma glucose level, and of at least 1 % in the HbA1c level, with hypoglycemic drug treatment. Systemic arterial hypertension and dyslipidemia are considered resolved when the patients do not need medication anymore, to maintain normal values for these conditions, while respiratory and orthopedic problems are considered resolved or improved based on patients perception of symptoms.
5.4.3 Early Surgical Results
The length of operative laparoscopic procedures ranged from 110 to 280 min (average 170 min). Mainly due to coverage restrictions, only 361 were laparoscopic procedures, and 659 procedures were performed with open or mixed access. In general, patients were discharged in the third POD. Early significant 30-day postoperative complications occurred in 60 patients (6 % Table 5.1). Nineteen patients required reoperation (1.9 %). There were 2 deaths (0.2 %).
Fistula—9a cases (0.9 %) Bleeding (requiring reoperation or blood transfusion)—8 (0.8 %) |
Intestinal subocclusion—8 cases (0.8 %) |
Non-obstructive prolonged ileus—7 cases (0.7 %) |
Symptomatic atelectasis or pneumonia—5a cases (0.5 %) |
Symptomatic partial portal thrombosis—5 cases (0.5 %) |
Acute crises of urolithiasis—5 cases (0.5 %) |
Early incisional dehiscence, in open cases—4 cases (0.4 %; 0.6 % of open cases) |
Intraperitoneal infection or abscess of unknown origin—3 cases (0.3 %) |
Cardiac complications—2 cases (0.2 %) |
Compression neuroplegia—2 cases (0.2 %) |
Clinically significant rhabdomyolysis—1 case (0.1 %) |
Pulmonary thromboembolism—1 case (0.1 %) |
5.4.4 Late Surgical Results
Late complications included 132 patients with cholelithiasis (21.9 %), 19 (3.1 %) with incisional hernias, and 15 (2.4 %) with internal hernias or intestinal subocclusion related to adherences. Most of these patients did not present the typical signs of obstruction, vomiting, or interruption of evacuation because of the TB. Pain was the predominant presenting symptom, along with some mostly left-sided abdominal distension. Postoperatively, three cases (0.5 %) had a hiatal hernia, corrected by a hiatoplasty due to gastroesophageal reflux. Approximately 35 % of patients were taking a daily or occasional proton-pump inhibitor (PPI) for heartburn. In relation to the frequency of bowel movements, mild constipation was rarely observed; most patients presented more frequent bowel movements, or maintained the same frequency observed before surgery. Typically, patients reported softened stools, and many reported a worsened odor in flatus and feces. However, the severity of these symptoms was rarely reported to be a problem, especially if the 120 cm common channel were used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree