© Springer Science+Business Media New York 2016
Marina Kurian, Bruce M. Wolfe and Sayeed Ikramuddin (eds.)Metabolic Syndrome and Diabetes10.1007/978-1-4939-3220-7_1212. Sleeve Gastrectomy
(1)
California Pacific Medical Center, 2340 Clay street, 2nd floor, San Francisco, CA 94115, USA
Keywords
DiabetesBariatric surgerySleeve gastrectomyGastric sleeveBilopancreatic diversion/Duodenal switchDiabetes resolutionIn the USA, there are over 100 million Americans with diabetes and pre-diabetes and over 72 million Americans with obesity. Two thirds of adult onset diabetes is directly associated with obesity.
Diabetes is a heterogeneous disease with three main types: type 1, type 2 and latent autoimmune diabetes. Obesity has been associated as a significant risk factor for the development of type 2 diabetes. Individuals with a body mass index > 35 kg/m2 are 20 times more likely to develop diabetes than those with a BMI < 25 kg/m2 [1]. The main condition that develops in type 2 diabetes is insulin resistance. In type 1 diabetes and LADA, lack of insulin production is the primary condition but one should be aware that many of these patients also develop a resistance to exogenous insulin if they become obese. It is generally well understood that any weight loss or improved dietary management can help to control diabetes. However, when these attempts prove ineffective, bariatric surgery is considered. In 1995, Walter Pories published an article in the Annals of Surgery titled, “Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus” [2]. Almost 20 years later, bariatric surgery has proven to be an effective treatment for obesity related diabetes. This chapter reviews the current role of the Sleeve Gastrectomy in the treatment of obesity related diabetes. Concepts reviewed include diabetes outcomes by BMI, duration of diabetes, time to resolution, C-peptide production, Pouch/Bougie size, and comparison to other procedures. The heterogeneity of diabetes and the variations in sleeve gastrectomy technique make it difficult to analyze outcomes but many reasonable conclusions can be made.
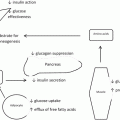
The sleeve gastrectomy (or gastric sleeve ) has emerged as an acceptable procedure for almost any bariatric patient. During the open bariatric surgery era, it was the restrictive component of the Duodenal Switch [3]. The advent of laparoscopic bariatric surgery facilitated the sleeve gastrectomy as a first stage, lower risk option in high risk patients [4]. In the last 5 years, it has proven to be a reasonable single stage option for the lower BMI group of patients and for patients with unique contraindications to adjustable gastric banding or intestinal bypass procedures [5, 6]. In addition, recent reports have revealed that the sleeve can yield durable diabetes improvement [7]. There is also some proof that removing the volume part of the stomach (greater curvature) also removes most of the cells that produce ghrelin , which may also contribute better than expected weight-loss results and diabetes outcomes in the absence of malabsorption [8].
Historically, Buchwald et al. [9] revealed a gradation of effect on diabetes resolution based on the procedure being purely restrictive versus having a large component of malabsorption. The Bilopancreatic Diversion/Duodenal Switch had a 98.9 % diabetes resolution whereas the purely restrictive Gastroplasty procedures had a 71.6 % diabetes resolution . It is difficult to extrapolate these outcomes to the sleeve gastrectomy but the pouch is generally smaller than with the duodenal switch and the gastric resection may contribute to diabetes resolution more than just a gastroplasty. At least it is reasonable to conclude that pure restriction can improve diabetes.
A 2009 systematic review of sleeve gastrectomy by Brethauer et al. [10] included 10 studies and 754 patients with follow-up on comorbidities. The overall remission rate for diabetes was 56 % with an additional 37 % demonstrating improvement. A 2010 systematic review by Gill et al. [11] including 27 studies and 673 patients revealed that diabetes resolved in 66.2 % and improved in 26.9 % of patients. Other small sleeve gastrectomy series have reported diabetes resolution rates from 80 to 88.9 % at 1 year [12–15]. Menenakos et al. [16], in a prospective single center study with 1 year follow-up confirmed a diabetes resolution rate of 84 % (30 of 36 diabetics). These early reports did not comment on the duration or severity of diabetes or differentiate between BMI groups or pouch sizes.
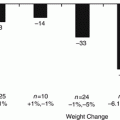
The results of sleeve gastrectomy on diabetes when associated with BMI are limited to date. Basso et al. [17] reported a diabetes cure rate of 69 % in a higher BMI group of patients (BMI 54 kg/m2) compared to 88 % cure in a lower BMI group (BMI 45 kg/m2). Both groups had improvement, but the lower BMI group achieved a superior result. Magee et al. [18] reported a very low diabetes improvement rate of only 23 % in a group of patients with a BMI > 60 kg/m2. Conversely, Abbatini et al. [19] reported a diabetes cure rate of 88 % (8/9) in nine low BMI (30–35 kg/m2) diabetics undergoing sleeve gastrectomy compared to 0 % cure rate for nine diabetics under medical treatment. The one diabetic that was not cured was diabetic for 20 years.
Diabetes resolution correlating with pouch size is controversial and may be more closely related to actual weight lost. Atkins et al. [20] reported a longitudinal retrospective study of 294 sleeve patients. 106 patients had a sleeve gastrectomy done using a 50 French bougie and 185 patients had it done using a 40 French bougie. Diabetes resolution was 5.2 times greater at 4 years postoperative with the smaller bougie. Similar outcomes were seen for dyslipidemia and hypertension. The %EBMIL was greater for the 40 French group (60.2 % compared to 45.4 %). Spivak et al. [21] used a retrospective case control study of 66 patients undergoing a sleeve gastrectomy with a 42 French bougie and 54 patients undergoing a sleeve gastrectomy with a 32 French bougie. Both groups had the antral resection start 1–2 cm from the pylorus. At 1 year, %excess weight loss was 67 and 65 % and diabetes resolution was 79 and 83 %. The difference was not significant. Abdallah et al. [22] reported the impact of the extent of antral resection in a prospective randomized study of 105 patients. Fifty-two patients had the antral resection start 2 cm from the pylorus and 53 patients had the antral resection start 6 cm from the pylorus. The group with the staple line starting 2 cm from the pylorus had significantly better weight loss (71.8%EWL at 2 years) compared to the 5 cm group (61%EWL at 2 years). There were only 16 diabetics in the study but the group with the smaller antrum had a diabetes resolution of 80 % (4/5) and the group with the larger antrum had a diabetes resolution of only 36.4 % (4/11).
Time to resolution of diabetes after sleeve gastrectomy has been variably reported. Rizzello et al. [23] reported on 17 diabetics early after sleeve gastrectomy and noted that within 5 days of surgery there was a reduction in glucose, insulin, and insulin resistance that persisted beyond 60 days. The diabetes cure appeared rapid and before weight loss occurs. Rosenthal et al. [24] reported on 30 diabetics undergoing sleeve gastrectomy at 2 and 6 months postoperatively. At 2 months, diabetes resolution was 27 % and at 6 months it was 63 %. The best resolution was in those with a shorter duration of diabetes and better weight loss. Casella et al. [25] reported on the duration of diabetes as a prognostic factor. A group of 40 sleeve gastrectomy patients with diabetes duration less than 10 years had a 100 % diabetes remission rate whereas a group of 16 sleeve gastrectomy patients with diabetes duration more than 10 years had only a 31 % remission rate. Additional reports provide more detail on time to resolution. Shah et al. [26] noted that a diabetes cure can take more than 1 month and up to 1 year. In a series of 53 diabetics, 81 % of patients were off diabetic medications at 1 month postoperative and 96 % at 1 year. Slater et al. [27] reported on a series of 22 diabetics with diabetes resolution of 62 % at 2 months and 75 % at 12 months. The duration of cure has been reported to range from 69 % at 3 years [28] to 100 % at 5 years [15] in studies with less than 25 patients. There are a myriad of reasons for the disparity in resolution between studies such as starting BMI, patient ethnic background, pouch size, duration of diabetes, etc.
There are reports comparing the time to diabetes resolution between the sleeve gastrectomy and the gastric bypass [14, 29–31]. They appear to have similar cure rates at 2, 4, and 12 months. Recent meta-analyses have confirmed these similar rates out to 3 years with only a slight advantage for the Roux-en-Y gastric bypass. Yip et al. [32] reported a systematic review and meta-analysis including 33 studies and 1375 patients. Diabetes resolution between the gastric bypass and the sleeve gastrectomy was compared. Unique to this meta-analysis was defining the remission criteria of a hemoglobin A1c of <6.5 %. Most studies previously mentioned considered diabetes resolved if the patient was off of medication. In this study, diabetes remission at 3 years postoperative was 81 % for gastric bypass and 80 % for sleeve gastrectomy. There was no significant difference in either diabetes resolution or weight loss between the gastric bypass and sleeve gastrectomy at 3 years. The other two meta-analyses [33, 34] confirm these findings with the exception that at some time points after surgery the gastric bypass may have slightly better weight loss and a slightly better resolution of diabetes—but neither is statistically significant. One study does report a much higher diabetes resolution rate for the RNY compared to the sleeve gastrectomy. Lee et al. [35] reported on 60 low BMI (25–35 kg/m2) diabetic in Taiwan with poor diabetic control (A1c >7.5). The diabetes resolution at 12 months for the RNY in this group was 93 % compared to only 47 % for sleeve gastrectomy patient group. This study does suggest that the RNY may be superior for the more severe diabetics. However, other similar outcomes have yet to be reported. 86 % of the patients in the study were RNY patients which may have skewed the conclusions. The authors also noted that the highest cure rates were in those with diabetes for less than 5 years and with BMI > 30 kg/m2 (presumably this group has more obesity related insulin resistance). Interestingly, all of these studies presumably represent a time point when the surgeon has already optimized the gastric bypass technique but may still be relatively early in the sleeve gastrectomy technique. These reviews suggest that this early sleeve technique is comparable to the more established gastric bypass technique. The sleeve gastrectomy diabetes resolution rates may continue to improve with surgeon experience and patient selection.
Comparative studies between the adjustable gastric band and the sleeve gastrectomy show the sleeve to have a superior diabetes resolution. Omana et al. [36] compared 49 sleeve patients with 74 band patients. There were 29 diabetics and 17 diabetics in each group, respectively. The sleeve group had a higher BMI of 52 kg/m2 compared to 44 kg/m2 in the band group yet the sleeve diabetes resolution was 100 % and only 48 % for the bands. Abbattini et al. [37] reported similar outcomes with a band diabetes resolution rate of 60.8 % compared to 80.9 % for the sleeve group.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree