Skin cancer of the head and neck is a heterogeneous group of malignancies that together constitute the most common cancer of this anatomic region. The majority of cases are nonmelanoma skin cancers (NMSC) such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), but melanoma and Merkel cell carcinoma (MCC) are increasingly encountered. Although most skin cancers of the head and neck are successfully treated by a variety of practitioners using an array of modalities, melanoma and aggressive variants of NMSC demand a multidisciplinary approach—the challenge lies in recognizing the dangerous lesions early in their course to facilitate comprehensive staging and treatment. Because melanoma is thoroughly addressed elsewhere in this text, this chapter will focus on NMSC, particularly BCC and SCC, in addition to the unique aspects of cutaneous melanoma of the head and neck.
About 3.5 million NMSC are diagnosed yearly in the United States, with BCC constituting roughly 80% of these cases.1 SCC accounts for about 20% of diagnoses, while MCC, rare adnexal carcinomas, and sarcomas comprise about 1% as well.2 The true incidence is hard to ascertain, as NMSC is infrequently reported to cancer registries, including the Survival Epidemiology and End Results (SEER) database. Health care expenditures related to NMSC exceeded $426 million per year by the mid-2000s in the US Medicare population alone—it may even reach $2 billion; costs are likely even greater in countries where these patients are more often treated in the hospital setting.3,4 Though less common than NMSC, cutaneous melanoma has been estimated to afflict over 76,000 people in 2013 and it accounts for over 70% of all deaths from skin cancer.5,6 The lethality of melanoma is widely recognized, but the mortality risk for aggressive NMSC, particularly SCC, is more difficult to quantify. Between 3900 and 8791 deaths are estimated to be annually attributable to cutaneous SCC (cSCC), with cSCC contributing to as many deaths as melanoma in the central and southern United States.7
Skin cancer is typically related to exposure to ultraviolet (UV) radiation, and 75% of NMSC arise on the head and neck because of the increased sun exposure of these areas.1 Interestingly, head and neck subsites only account for 15% to 21% of cutaneous melanoma; evidence suggests a worse prognosis for head and neck sites, particularly the scalp and neck.8,9 Both NMSC and melanoma are increasing in frequency, and the at-risk populations share some demographic features. Although Caucasian men over age 50 are at greater risk of developing NMSC, there is evidence that the disease is becoming more common among younger patients of both genders and women, and that aggressive variants manifest in these subpopulations as well.10,11 Similarly, cutaneous melanoma most commonly afflicts Caucasian men aged 55 to 64, but head and neck melanomas tend to occur more commonly in patients over 70, reflecting the potential impact of cumulative sun exposure in tumorigenesis.12,13
Ultraviolet light exposure, including both UV-A (320 to 400 nm) and UV-B (290 to 320 nm), from sun exposure is the chief risk factor for the development of both NMSC and melanoma. UV-B damages keratinocyte DNA via pyrimidine dimer formation in the p53 tumor suppressor gene, as well as loss of the fas-fas ligand interaction. UV-A exposure leads to DNA damage through oxidative stress. The resulting genomic instability facilitates the acquisition of additional mutations in driver oncogenes, such as H-ras, and promotes the progression through premalignant lesions known as actinic keratoses (AKs), noninvasive cancer (SCC in situ), and finally cSCC.14 Although UV light is also linked to BCC development, activation of the Hedgehog pathway, along with the ability to escape immune system surveillance, are integral to progression of BCC.15,16
Melanin production by melanocytes serves to protect the deeper layers of the skin from UV radiation penetration, so fair skinned individuals are at elevated risk for developing skin cancer because they produce less melanin. Both intense intermittent sun exposure leading to blistering sunburn and prolonged sun exposure are associated with the development of NSMC.17 The impact of sun exposure is further underscored by a doubling of the incidence of SCC with every 8 to 10 degree change in latitude closer to the equator, and by increases in NMSC in medium- or high-UV index states.18,19 The risks of UV exposure are not limited to natural sunlight—there is emerging evidence linking tanning bed use (which predominately use UV-A light) to NMSC and melanoma development.20,21
Immune compromise places patients at an increased risk of developing NMSC, and immunocompromised patients tend to develop more SCC than BCC, flipping the 4:1 ratio seen in immunocompetent individuals. Patients with human immunodeficiency virus (HIV) infection have a twofold higher incidence of SCC than HIV(−) individuals, and this risk increases with the degree of immunosuppression.22 Patients with chronic lymphocytic leukemia, non-Hodgkin’s lymphoma, and other immunodeficient states, including drug-induced immunosuppression, are also at elevated risk.
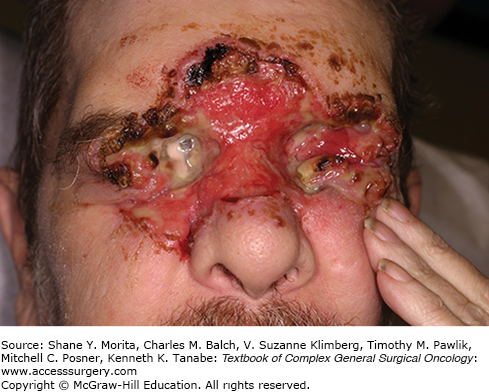
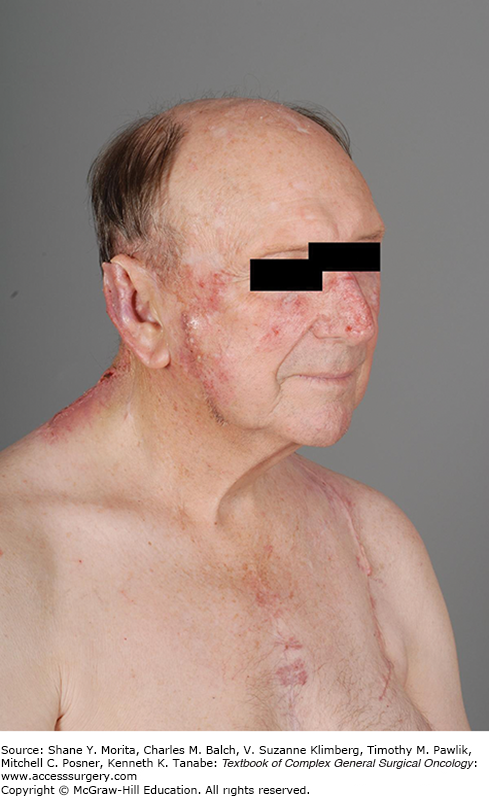
Perhaps the most challenging cohort of immunocompromised individuals with regard to skin cancer consists of solid organ transplant recipients. NMSC is the most common post-transplant malignancy, and these tumors are more likely to metastasize and lead to increased morbidity and mortality, as depicted in Figure 52-1.23 The 10-year incidence rate of NMSC for liver transplant patients ranges from 10% to 26%, and patients undergoing kidney-pancreas and heart transplants are at higher risk than kidney transplant patients, whose 10-year incidence of SCC is commonly reported as 13% to 36%, but there is variation among patient populations and geography.24 Patients who are older at the time of transplant develop the lesions at short interval, and patients with an incident NMSC will suffer at least one additional lesion within 5 years. This elevated risk of NMSC has been attributed to not only the degree of immunosuppression, but also the immunosuppressive drug regimens, with azathioprine, calcineurin inhibitors such as cyclosporine and tacrolimus, and anti-metabolites like mycophenolate mofetil (MMF) more closely associated with NMSC development than m-TOR inhibitors, including sirolimus.24 Conversion to m-TOR inhibitors has actually reduced recurrence rates and delayed or decreased the appearance of new lesions without increasing acute rejection risk, but the impact of this conversion on disease-free survival remains unclear.25,26
FIGURE 52-1
This 72-year-old male is 10 years removed from an orthotopic heart transplant. Beginning 6 months after his transplant, he has been treated for over 34 cutaneous squamous cell carcinomas and at least 14 basal cell carcinomas. He bears the stigmata of multiple resections, countless episodes of cryotherapy, parotidectomies, neck dissections, complex reconstructions, and even multiple episodes of radiation therapy.
Molecular and genetic factors contribute to the development of NMSC. Xeroderma pigmentosum is an autosomal recessive syndrome in which defective DNA repair leads to the development of numerous, aggressive BCC, SCC, and melanoma in childhood. Congenital basal cell nevus syndrome (Gorlin’s syndrome) is an autosomal dominant disease defined by multiple pigmented BCC, palmar pits, odontogenic keratocysts, rib abnormalities, and calcification of the falx cerebri.27 Additional risk factors for developing NMSC of the head and neck, including additional genetic conditions, exposures, and other patient characteristics are listed in Table 52-1.
Risk Factors for Skin Cancer of the Head and Neck
| Risk Factors for NMSC | Risk Factors for Melanoma |
|---|---|
| Environmental/exposure factors | Environmental/exposure factors |
|
|
| Patient factors | Patient factors |
|
|
|
|
|
|
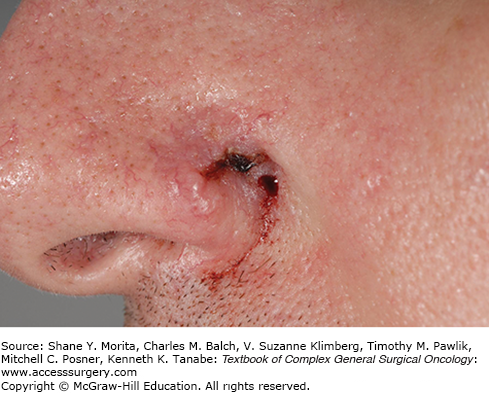
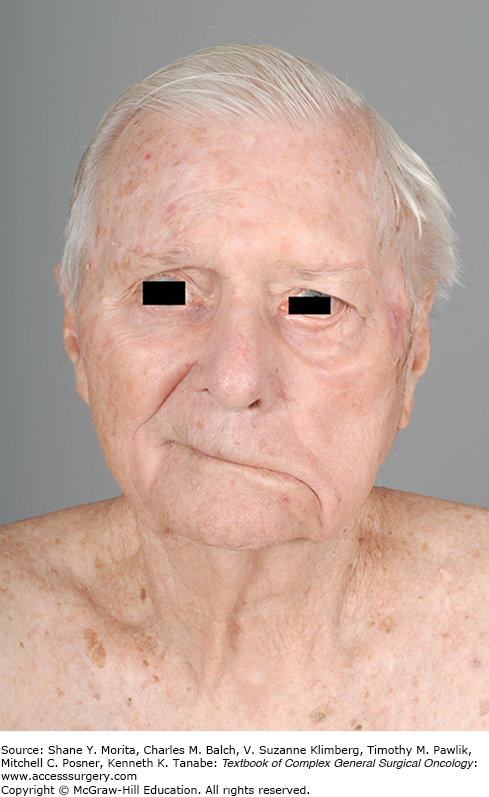
Basal cell carcinoma constitutes the majority of cases of NMSC, and there are several morphological subtypes including nodular, superficial, micronodular, infiltrating, sclerosing, fibroepithelial, and others. The nodular type is the most common, presenting as the characteristic “rodent ulcer” with raised, rounded edges, superficial telangiectasias, and a central ulceration, as depicted in Figure 52-2. These lesions are characterized by nests of malignant cells with peripheral palisading nuclei.
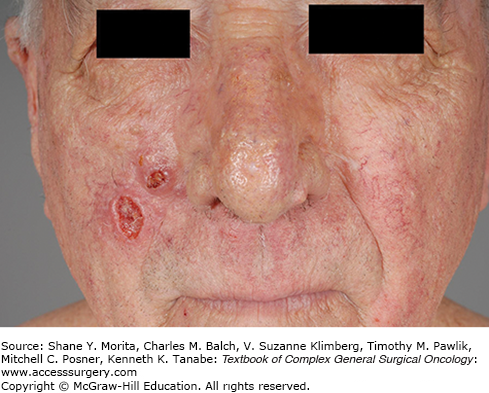
Infiltrative BCC, historically known as morpheaform BCC, often has significant subclinical spread and contains multiple islands or poorly defined projections of tumor (Fig. 52-2).28 Both infiltrative and micronodular BCC may behave more aggressively, since they may invade more deeply and are more difficult to clear surgically. A single BCC may exhibit more than one histological pattern, and therapy must be directed at the more aggressive component, as in the case of basosquamous (or metatypical) carcinoma.15 Although BCC rarely metastasizes, neglected lesions may be locally destructive, requiring radical therapy for clearance and resulting in significant morbidity such as the patient in Figure 52-3.28
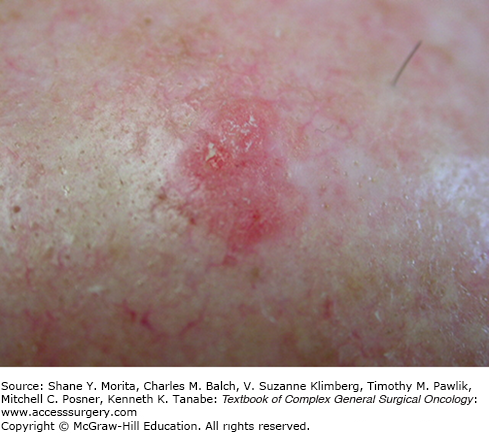
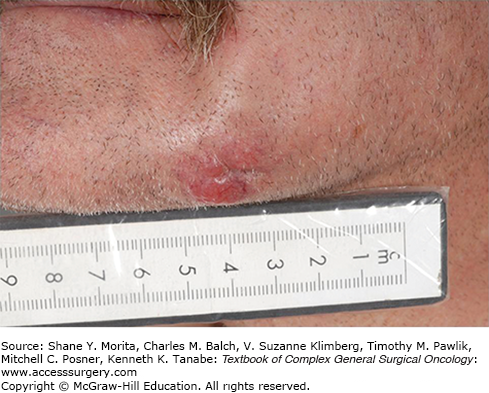
Cutaneous SCC often has a defined precursor lesion, the AK, and once manifest, it generally behaves more aggressively than BCC. Several subtypes of cSCC exist, including verrucous, spindle cell, desmoplastic, and basosquamous variants. The degree of differentiation is also clinically important. AKs are hyperkeratotic, scaly plaques or papules that may be erythematous, flesh-colored, or pigmented (Fig. 52-4)—they have an annual risk of progression to malignancy that is estimated at 0.075% to 0.096% per lesion per year without any reliable predictors of which AK will progress.29 Keratoacanthoma (KA) is a rapidly growing nodule with a central keratin core that often involutes after 2 to 6 months, but it is clinically challenging because it histologically resembles well-differentiated SCC.30
Invasive SCC is composed of cords and nests of atypical squamous cells arising from the epidermis and invading into the dermis; focal keratinization and variable degrees of differentiation are possible. Clinically, SCC appears as plaques or nodules, often with ulceration or even keratin horns. Poorly differentiated SCC (Fig. 52-5), spindle cell SCC, desmoplastic SCC, and basosquamous SCC frequently demonstrate aggressive behavior, and these variants are more prone to nodal metastases, perineural invasion (PNI), and recurrence.30
FIGURE 52-5
Recurrent, poorly differentiated SCC of the right melolabial fold in a 78-year-old man who had previously undergone Mohs surgery. He was treated with revision Mohs surgery and sentinel lymph node biopsy, with identification of metastatic SCC in a parotid lymph node. After refusing comprehensive lymphadenectomy, he underwent radiation therapy to the primary site, parotid, and cervical lymphatics, remaining NED at 30 months.
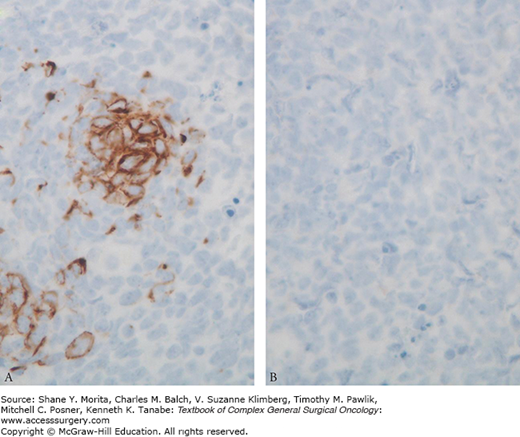
Merkel cell carcinoma is uncommon cutaneous malignancy that presents as a rapidly growing pink to purple nodule, typically in sun-exposed areas (particularly the head and neck), of older Caucasian men (Fig. 52-6). Diagnosis of MCC is often challenging because tumor cells appear as small, round cells predominately arising from the dermis with a high mitotic index. Although initially thought to arise from a dermal mechanoreceptor, the Merkel cell, recent evidence suggests that MCC arises from a primitive epidermal stem cell.31 The paranuclear staining pattern on immunohistochemistry to CK20 (Fig. 52-7) is essentially pathognomonic for MCC. MCC often afflicts immunocompromised patients, and there is evidence of a viral etiology since Merkel cell polyomavirus has been detected in 75% to 89% of cases.32 Diagnosis of MCC may be suspected based on AEIOU clinical features: A, asymptomatic and painless; E, expanding rapidly; I, immunosuppression; O, patient older than 50; U, UV-exposed areas.33 Local recurrence, regional metastases, and distant failure are common, so multidisciplinary treatment is paramount. Its increasing frequency and diagnostic and treatment challenges merited a unique staging system in 2010 from the American Joint Committee on Cancer (AJCC).34
Although the vast majority of NMSC consists of BCC and SCC, other types are also encountered, albeit rarely. Low-grade sarcomas such as dermatofibrosarcoma protuberans and high-grade sarcomas like angiosarcoma are encountered on the skin of the head and neck.35,36 Furthermore, adnexal structures of the skin may give rise to sebaceous carcinoma, microcystic adnexal carcinoma, and pilomatrix carcinoma, as well as other rare histologies. These lesions present unique demands, and surgery often constitutes a key element of the multidisciplinary approach.37
Surgeons must maintain a high index of suspicion when evaluating a patient with a cutaneous lesion, as both melanoma and NMSC treatments may be associated with significant morbidity and indeed mortality. A detailed history should elicit potential recreational or occupational exposures, use of sun-protective behaviors (if any), past history of skin lesions, a family history of skin cancer, and comorbid conditions, especially transplant status and immunosuppression. Several questions are fundamental, and these are listed in Table 52-2. The presence of formication (the sense that insects are crawling under the skin) is worrisome for PNI and warrants closer investigation with anatomic imaging.
Focused NMSC History
|
Patients presenting with a neck or parotid mass should be queried for any antecedent history of skin lesions or malignancy. The patient may not know that a particular lesion was cancer, or that it could metastasize, so it is imperative to ask whether there have been any skin lesions previously treated about the head and neck. Metastatic skin cancer may present in the parotid gland, and patients with parotid lesions should be asked if they have been treated for an ipsilateral skin lesion, especially in the temporoparietal and periauricular regions.38 Interestingly, the most common parotid malignancy in Australia is metastatic skin cancer.39
After identifying potential risk factors for NMSC in the history, care must be taken to note the general skin phototype of patients, historically based on the Fitzpatrick scale; types I and II skin (light skin, light eyes, and a propensity to burn rather than tan) are more prone to sunburn and are more closely linked to the development of skin cancer.40,41 The lesion in question should be closely examined, focusing on its location and relationships to adjacent structures such as the orbit or ear canal. Intranasal examination, including nasal endoscopy, may identify full thickness involvement of the nose. Assessment of the oral mucosa is indicated in larger, thick cheek lesions, and in all lip masses. Lesions in the H-zone of the face, including the midface, upper lip, nose, medial canthus, eyelids, temples, lateral forehead, malar eminences, preauricular cheek and periauricular tissues, are considered more difficult to treat because of a high risk of local recurrence and a tendency for deep invasion.42 Suspicious findings should be measured in the lateral dimension, followed by palpation to ascertain the potential for involvement of underlying structures. For pigmented lesions, dermoscopy is a useful adjunctive technique that has been shown to improve the sensitivity of early melanoma detection.43
A detailed cranial nerve exam is imperative because PNI of skin cancer, particularly SCC, occurs in up to 5% to 10% of patients, although it is usually an incidental finding on pathology.44 Because the trigeminal nerve and the facial nerve are most commonly involved, focused assessment of sensation in all branches of the fifth cranial nerve and an assessment of movement in all branches of the facial nerve is required.45 PNI often presents as severe pain in one branch of the trigeminal nerve or as a slowly evolving paralysis of one branch of the facial nerve that progresses over time to complete facial paralysis—despite the obvious clinical difference, it is commonly misdiagnosed as Bell’s palsy, which is the sudden onset of complete facial paralysis (Fig. 52-8).
FIGURE 52-8
Complete left facial paralysis related to perineural invasion of the facial nerve. Note the smooth forehead with lateral hooding of the eye, scleral show from laxity in the lower lid, drooping melolabial crease, and twisted corner of the mouth. This clinical picture evolved slowly, with gradual and progressive paralysis of individual nerve branches, making it a distinct clinical picture from Bell’s palsy.
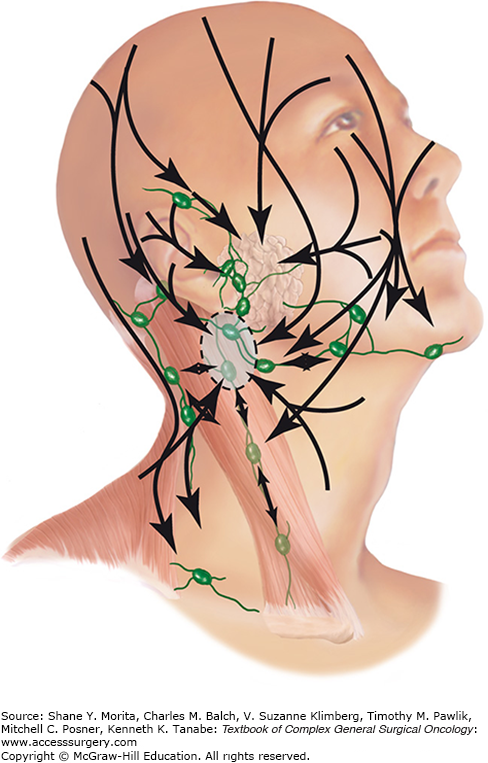
Palpation of the parotid glands and cervical lymphatics, including those in the posterior triangle and suboccipital regions is indicated as well. Although the head and neck is known for its variable or challenging lymphatic drainage, patterns exist: cutaneous lesions of the lateral face, periauricular region and temperoparietal scalp tend to metastasize first to the parotid bed (which is essentially a lymphatic basin with a robust array of intraglandular and capsular lymph nodes) and upper jugular nodes, and central midface lesions have the potential for bilateral anterior cervical metastases (Fig. 52-9).38,46 For patients who present with a parotid or neck mass, a thorough inspection for a synchronous skin primary should be undertaken, along with an examination of the upper aerodigestive tract, as in the case of an unknown primary.
FIGURE 52-9
The lymphatic drainage of the skin of the head and neck. Note the importance of the parotid bed and the upper jugular lymph nodes for numerous cutaneous subsites. (Reproduced with permission from Vauterin TJ, Veness MJ, Morgan GJ, et al. Patterns of lymph node spread of cutaneous squamous cell carcinoma of the head and neck, Head Neck. September 2006;28(9):785–791.)
Suspicious cutaneous lesions of the head and neck merit incisional or narrow-margin excisional biopsy, as appropriate anatomically. Incisional biopsy may be accomplished with a 2-, 3-, or 4-mm punch for a full-thickness assessment of the involved skin. Shave biopsy is an acceptable modality in NMSC, provided that adequate depth can be determined. The ideal pathology report provides not only a pathologic diagnosis, but also provides information about depth, differentiation, and vascular or perineural invasion. Unfortunately, synoptic reporting is not commonplace in NMSC, unlike melanoma, and this lack of standardized reporting on biopsy specimens may frustrate the early identification of aggressive NMSC.47
The vast majority of cases of NMSC require no imaging studies in their initial workup and management. However, certain findings in the history and physical merit further evaluation. Larger lesions and clinically thick or fixed lesions in the cheek and preauricular region have the potential to involve the parotid gland or Stensen’s duct, and a contrast CT scan of the neck may identify parotid extension and facilitate adequate preoperative planning and comprehensive resection (Fig. 52-10). CT scans also may identify invasion into underlying bone, cartilage, muscle, and fascia, as well as bony remodeling or enlargement of neural foramina and canals in cases of suspected PNI. However, MRI is more sensitive than CT for detecting early perineural spread.48 MRI and CT often provide complementary information in patients with deep scalp lesions, as the CT scan may demonstrate bony destruction and the MRI may depict the extent of intracranial spread. Clinically suspicious parotid masses or cervical lymph nodes also benefit from anatomic imaging with MRI, CT scan, or comprehensive neck ultrasound to permit accurate nodal staging. Nodes larger than 1.5 cm in levels I and II and those larger than 1 cm in other neck levels may warrant additional evaluation with fine needle aspiration biopsy. Routine use of PET-CT in head and neck NMSC is not recommended, except in MCC, where initial staging PET scans impacted treatment in 37% of cases, including 17% where the disease was upstaged.49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree