Introduction
Nociceptor cell bodies are located in the trigeminal ganglion or the dorsal root ganglion, and their nerve endings terminate in the skin. These nerve fibers are present in nerve bundles in the subepidermal neural plexus and some of the free nerve endings penetrate the epidermal–dermal layer. Damage to the nerve fibers due to diabetes may give rise to symptoms or signs of diabetic neuropathy (DN). The damage manifests typically as a decrease of the unmyelinated or thinly myelinated nociceptive nerve fibers in the skin, but other morphological abnormalities can also be seen. This loss is quantified by taking a small punch skin biopsy (typically 3 mm in diameter) and by estimating the density of nerve fibers penetrating the epidermal layer (the topmost layer of the skin ), frequently referred to as intraepidermal nerve fiber density (IENFD). This morphometric analysis and the decrease of IENFD are often considered being the pathological gold standard of small fiber damage when diagnosing DN, as it is the method that offers the most reliable, quantitative, and objective analysis of (small fiber) neuropathy. In fact, DN was one of the first diseases to be shown to have decreased IENFD as shown with skin biopsy . The skin biopsy method is only suitable to assess unmyelinated or thinly myelinated nerve fibers. To detect damage to the myelinated fibers, neurophysiological examinations such as nerve conduction studies (NCS) should be used, which, in turn, is unable to detect damage or loss of unmyelinated fibers.
The presence of nociceptor nerve endings in the epidermis has been known for over 150 years, but they were first described in 1868 . The first study to visualize the epidermal nerve fibers was published in 1989, where the authors collected skin samples from themselves and immunostained frozen sections using protein gene product 9.5 (PGP 9.5) that is a protein expressed on the nerves . It was, however, only in this century it became possible to easily and reliably immunostain the nerve fibers . Since then, the staining protocol has been optimized, general counting rules have been established, and the skin biopsy technique and the quantification of IENFD have been introduced in clinical practice, with normative reference values available at the distal leg, both for bright field and indirect immunofluorescence microscopy .
While the skin biopsy is considered minimally invasive, there is a less invasive alternative to skin biopsies to assess the IENFD. The “skin blister” method, where negative pressure vacuum is used to form a blister on the skin, can be performed to separate the epidermis from the rest of the skin . Studies have shown that there is a minimal difference of the IENFD values between the two methods and a high correlation between them . However, while the skin blister method is less invasive, there are some disadvantages, including that it is time consuming as it can take up to 90 minutes, and because it is superficial in nature, it is not possible to perform morphological analysis of the deeper skin tissue, for example, the dermis. Therefore, skin blisters are not frequently used, neither in clinical practice nor for research purposes.
Advantages and disadvantages of skin biopsies
Taking a skin biopsy is a process that only takes a few minutes, it is minimally invasive with the resulting wound heals in 7–14 days, the procedure can be repeated to follow disease progression over time, and gives reproducable and reliable results. The procedure is therefore being used more and more as a diagnostic tool in the clinic. Additional advantage of skin biopsies is the variability of different immunostainings and information that can be extracted from them. While quantification of IENFD is the gold standard, given that reliable antibodies are reliable, there are no limitations of alternative immunostainings that can be performed. Some of the analyses may hold the potential to explain why some patients with diabetes develop symptoms of DN, such as sensory dysfunction or neuropathic pain. There are also a number of morphological alterations that can be assessed and quantified, including axonal swellings or branching density . Skin biopsies and especially IENFD analysis have been used to follow efficacy of interventions by taking repeated biopsies, for example, during lifestyle changes .
However, there are a number of disadvantages to the skin biopsies and IENFD analysis. These include a low correlation between IENFD and other (more subjective) test results, such as clinical symptoms reported by the patients or measures from quantitative sensory testing (QST). In addition, IENFD analysis is unable to determine the etiology of the neuropathy . Low IENFD is seen in different etiologies of neuropathy, possibly due to a final pathway that is shared by all neuropathies regardless of the underlying disease, or that we are yet to discover a suitable marker capable of discriminating between different etiologies . The lack of correlation between IENFD and other measures such as symptoms or QST may either be due to the fact that while IENFD is an objective measure, symptom-reporting is highly subjective and QST is psychophysical in nature, or because IENFD analysis only quantifies damage in the peripheral nervous system and not in the central nervous system, which also plays an important role in sensory and pain perception.
The main disadvantages of the skin biopsies are perhaps that immunohistological stainings are difficult to standardize, and processing and staining protocols are heterogeneous. This means that fixation methods, staining protocols, and section thickness often vary between laboratories, making it difficult to compare results between laboratories. Quantification of IENFD or other measures is also subjective in nature and the quality of the quantification depends both on the quality of the tissue and the staining, and on the person performing the analysis. It is therefore important that the entire process is as standardized as possible. One way to ensure this is to follow a published and recommended guideline from the European Federation of Neurological Societies (EFNS) and the Peripheral Nerve Society (PNS) . This is especially important for diagnostic purposes and when comparing results from patients with already available normative reference values obtained following the guidelines. For research purposes, there can be various reasons for deviating from the guidelines. These should be described and the findings should not be compared with results obtained using other protocols. Ideally, each skin biopsy laboratory should have their own dataset with normative reference values for both sexes and each age, divided by decade, as IENFD decreases with older age and male sex.
IENFD quantification in nondiabetic etiologies
Quantification of IENFD can be used to assess nerve fiber damage in neuropathies of nondiabetic etiologies as well. Examples include hereditary neuropathies (e.g., Fabry disease and hereditary sensory neuropathy type 1 ), autoimmune neuropathies (e.g. Sjögren’s syndrome ), entrapment neuropathies (e.g., carpal tunnel syndrome ), neuropathy of infectious (e.g., HIV ), or toxic causes (e.g., following chemotherapy or alcohol-induced neuropathy ), in fibromyalgia and in idiopathic cases with no identifiable cause . In rare cases, a sural or peroneal nerve biopsy can be acquired. The nerve biopsy was often used as a diagnostic tool before the skin biopsy became widely available, but is now only used if it adds needed information and other tests are unable to provide, for example, in amyloidosis, atypical chronic inflammatory neuropathies and in nonsystemic vasculitis, where nerve biopsy is the only way to make a definite diagnosis .
How and where to take a skin punch biopsy following EFNS/PNS guidelines
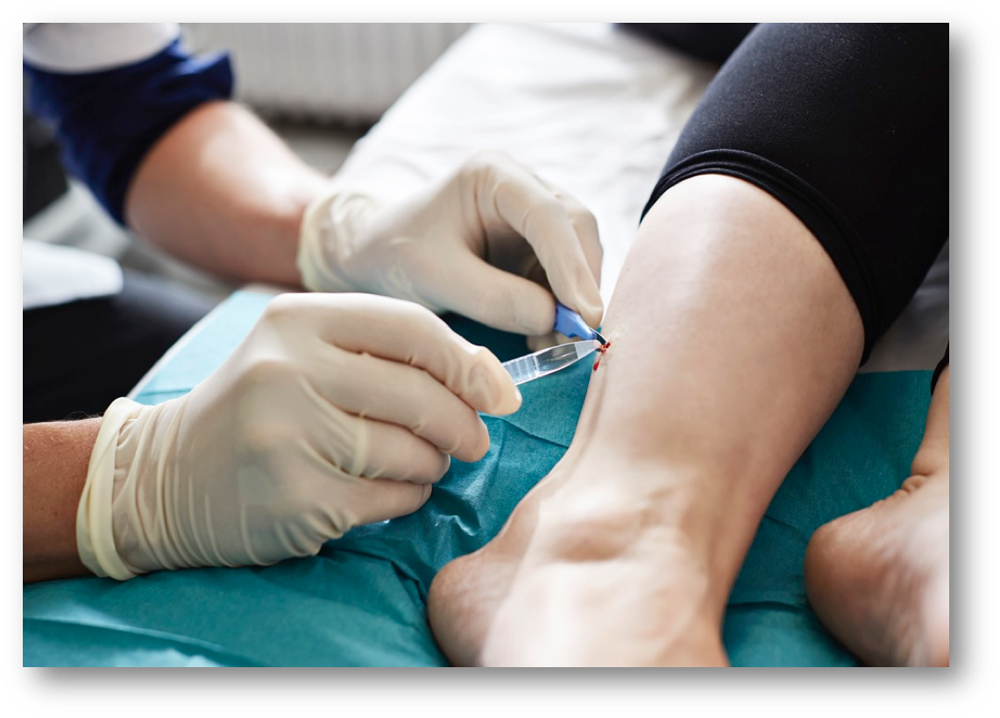
Skin biopsies can be taken from practically anywhere from the body, for example, where the patient complains about pain or other symptoms. For diagnostic purposes and in majority of research studies, the gold standard is to take a skin biopsy from the distal leg, 10 cm above the lateral malleolus (see Fig. 5.1 ).
Here we describe where the biopsies should be taken and how they should be processed when following the recommended EFNS/PNS guidelines .
The biopsy should be taken following local anesthesia using 0.5–1.0 mL lidocaine, under sterile conditions using 3–5 mm disposable biopsy punch. The biopsy should be immediately transferred to a fixative. There are several types of fixatives available, but both the type of fixative and the incubation time may influence the subsequent immunohistological staining. The biopsy should be fixed in either Zamboni’s fixative (2% paraformaldehyde, picric acid) or using freshly made 2% periodate-lysine-paraformaldehyde. Very importantly, the biopsy should be fixed over-night at cold temperature (4°C), but the fixation should not exceed 24 hours to avoid over-fixation, which will influence the subsequent immunohistological stainings. After fixation, the biopsy should be washed in either neutral buffer like PBS or in cryoprotectant for 2×5 minutes and then stored at cold temperature in cryoprotectant over night.
Freezing the biopsy
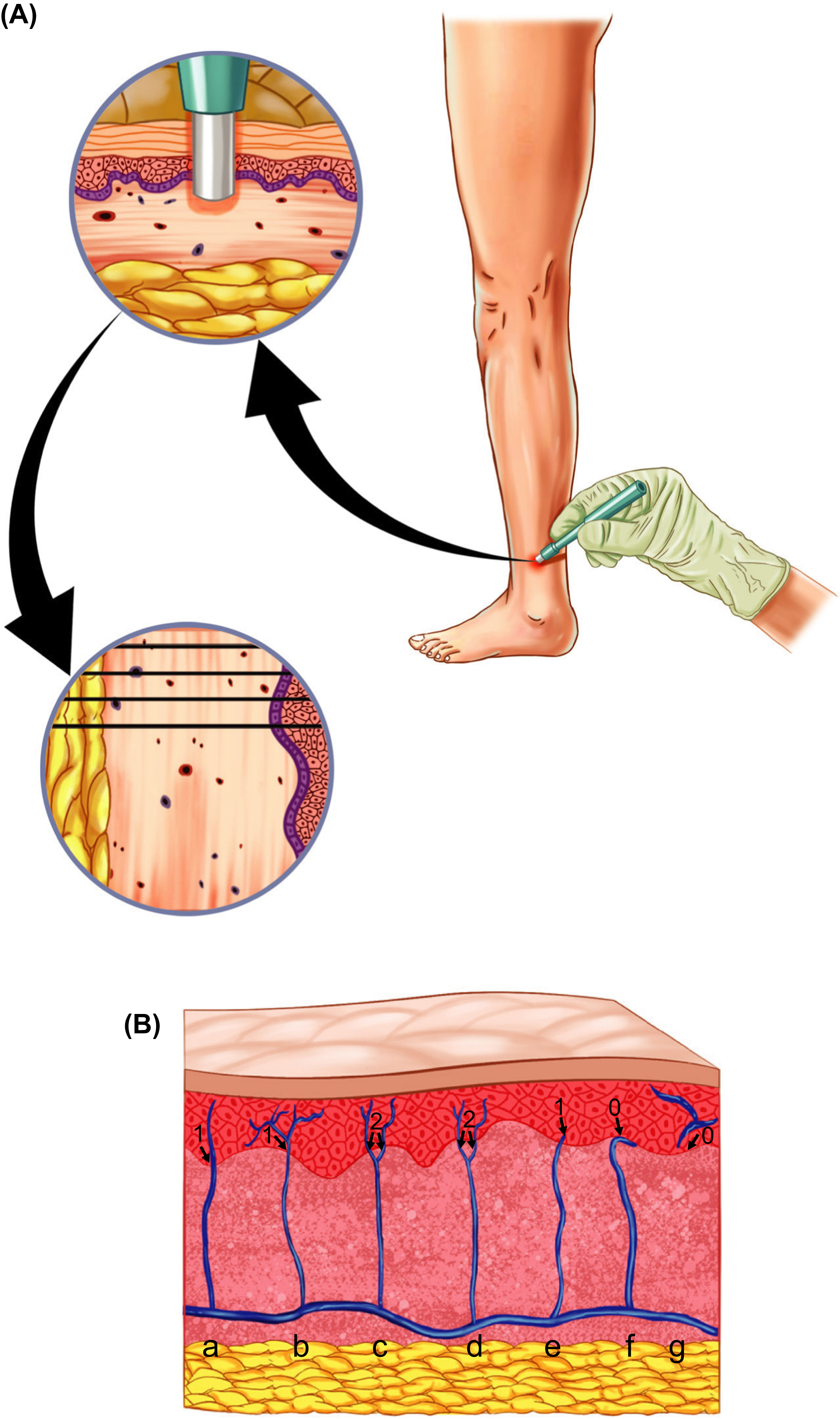
The biopsy is now ready to be frozen down. Freezing methods vary, but many laboratories use a cryostat. The biopsy should be frozen down submerged in optimal cutting temperature compound lying on the side so both epidermis and dermis are facing upward (see Fig. 5.2 ) and then stored at −20°C for a short-term storage (<1 year) or −80°C (>1 year). The biopsy can be sectioned using a cryostat where 50 µm thick sections are cut so that both epidermis and dermis are cut. At least three sections should be cut and they transferred directly to a neutral buffer (e.g., PBS) in a tissue culture plate allowing a free-floating staining, ensuring that both sides of the sections are exposed to the reagents used during the staining protocol. This is important as the section thickness is too thick for a fixed staining on slides with a risk of incomplete penetration. The sections can either be immunostained using immunohistochemistry or immunofluorescence. While there now exist normative reference values for both methods, immunohistochemistry is more frequently used, especially in the clinic. Immunofluorescence staining is typically used when performing more advanced stainings, for example, when using other antibodies than PGP 9.5 for IENFD analysis and when performing double- or triple stainings. Below is a description of a commonly used immunohistochemistry protocol for staining of skin biopsy sections for IENFD analysis.
Staining protocol for IENFD analysis
The first step of the staining protocol is at least 1 hour of blocking incubation using Tritron X-100, powdered milk, and normal serum made in the same animal as the secondary antibody that will be used later in the protocol. After three times wash, the skin sections are incubated in PGP 9.5 as primary antibody overnight on a slow-rotating shaker table at 4°C. Following another round of three times wash, the skin sections are incubated in secondary antibody for 1 hour at room temperature. The antibodies can be diluted in a weak blocking buffer. The sections are washed three times and then submerged in a solution containing 33.3% methanol and 3.3% hydrogen peroxide diluted in PBS for 30 minutes. After another round of wash, the biopsies are incubated in avidin–biotin complex for 1 hour and lastly, the nerve fibers are visualized using a peroxidase substrate kit until desired darkness has been reached. How long time that takes depends on the concentrations of the primary and secondary antibodies. It is important that the incubation time is long enough to visualize even the thinnest nerve fibers, but not too long to avoid high background staining that may cover the fibers and make them difficult to count or even make them invisible. The sections are submerged in gelatine solution and lined on a slide to air dry. The last step of the staining protocol is to briefly counterstaine the sections (e.g. in eosin), dehydrate them and then coverslip.
IENFD quantification
The IENFD quntification should be performed at high magnification using high power objective of at least 40X under live microscopy, to ensure that all unmyelinated nerve fibers are clearly visible. Quantification under lower magnification increases the risk of not seeing all nerve fibers entering the epidermis. The EFNS/PNS guidelines also include a set of “counting rules” to ensure uniformity between laboratories when counting. As the name suggests, only dermal nerve fibers that cross the epidermal–dermal boarder should be counted. This means that fibers that are only visible in either the dermis or the epidermis should not be counted (see Fig. 5.2 ). Because the section thickness is 50 µm, which is relatively thick, it is important to analyze the entire thickness ( Z -axis) of the section, which requires focusing up and down through the entire section thickness. The nerve fibers are so thin that the individual fibers are visible at different depths and not all fibers are visible at the same time or depth. Nerve fibers that branch from another nerve are also counted, but only if the branching point is located in the dermis and the branching fiber crosses the epidermal–dermal boarder (see Fig. 5.2 ). The total number of counted nerve fibers is divided with the length of the outer skin layer (measured in millimeters) to estimate the IENFD (expressed as number of fibers per mm). It is recommended to estimate the IENFD in at least three sections and to take the mean to obtain a more precise estimation.
Diagnostic accuracy of IENFD analysis in DN
Skin biopsies, just like NCS, are not often needed in patients with diabetes who meet the clinical DN criteria, for example, the one proposed by the American Diabetes Association (ADA), which is “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes” . Another often used classification is the Toronto classification from 2010, which divides DN into three levels of certainty of the diagnosis: possible, probable, and definite .
Quantitative information on nerve fiber damage is not needed in clinical practice in cases where there is no doubt about the diagnosis. Neither skin biopsy nor NCS can confirm or identify the underlying cause of the neuropathy and nor do they influence treatment strategies . Even so, IENFD is the pathologic gold standard of small nerve fiber damage in DN and is an important diagnostic tool in cases where there is uncertainty about the neuropathy-diagnosis. IENFD analysis has generally been shown to have good diagnostic sensitivity and specificity in DN, with good positive and negative predictive values . Using the Toronto definition of probable neuropathy, area under the curve analysis for IENFD to detect neuropathy has been reported to be 0.76, and slightly higher for SFN or 0.82 . However, a study that estimated the diagnostic utility of IENFD analysis and NCS in 366 patients with diabetes using several gold-standard definitions to determine the sensitivity and specificity found that IENFD analysis had a sensitivity of 81% but a specificity of 41%, while NCS had lower sensitivity of 40% but higher specificity of 95%. It is unclear if small fiber damage precedes large fiber damage, but that hypothesis may explain these differences. Devigili and colleagues compared the diagnostic utility of skin biopsy, QST, and clinical examination for neuropathies, and found that IENFD analysis outranked other tests with both sensitivity and specificity rates of around 90%, in patients with mixed etiologies . Similar comparisons have been made between IENFD analysis from skin biopsy and corneal confocal microscopy (CCM), where the tests showed strong and comparative findings. IENFD analysis and CCM are therefore promising methods, albeit bigger and more robust longitudinal studies are needed to truly reveal their clinical usefulness . The diagnosis of DN cannot, in other words, rely on a single test, but on clinical judgment using several tests including clinical history and examination. Apart from the usual biopsy site at the distal leg, an additional biopsy can be taken from the lateral proximal thigh (7–10 cm below the greater trochanter) to determine if the nerve damage is length-dependent or nonlength-dependent . DN most often presents as length-dependent where the distal leg biopsy shows lower IENFD compared with the biopsy from the lateral proximal thigh.
One advantage of skin biopsies is that they can be repeated over time, for example, to follow disease progression. Even so, not many longitudinal studies have been performed with IENFD analysis as an outcome. A study in 2016 compared the longitudinal rate of IENFD decline in healthy controls, in DN, patients with impaired glucose tolerance and in patients with idiopathic small fiber neuropathy . The study participants were evaluated at baseline and again 24–39 months later. The study found that the IENFD was reduced in all three neuropathy groups at all three biopsy sites along the leg, but not in healthy controls. The yearly rate of IENFD decline was estimated to be −1.42, −1.59, and −2.8 fibers/mm for the distal leg, distal thigh and proximal thigh, respectively, for the patient groups. A similar study from 2012 took biopsies from DN patients at baseline and again after 6 months to identify biomarkers to follow disease progression and, in future studies, to assess efficacy of disease-modifying agents . The study found that intraepidermal and subepidermal nerve fibers were reduced in DN for PGP 9.5 and transient receptor potential cation channel subfamily V member 1 (TRPV1) at both time points and that the annual rate of IENF loss was −3.76 fibers/mm (±1.46 fibers/mm).
Relationship between skin biopsy outcomes and symptoms or signs of DN
As mentioned earlier, there is only a low association between IENFD and presence of pain and especially pain intensity. A systematic review from 2019 looked into this relationship, and noted that of all neuropathy etiologies, DN is the most studied entity or a total of 40 studies at that time . The review included studies who reported at least one finding from skin biopsy and at least one additional parameter related to symptom or sign of neuropathy or nerve fiber function. The review reported that only eight out of 18 of the included studies that reported correlation analysis between IENFD and patient-reported pain found a significant inverse association, for example, lower IENFD with higher pain intensity. Of these eight studies, there were three studies that reported association between IENFD and patient-reported pain, one of which found a significant negative association. In addition, less than one-third of the studies found a significant association between IENFD and pain intensity. It seems therefore unlikely that low IENFD determines the presence or severity of painful symptoms in DN, even if it is a key pathological finding. In terms of association between IENFD and QST measures, the review reported that 22 out of 29 studies (76%) found significant association between IENFD and at least one QST parameter. However, when solely looking at studies that included DN or impaired glucose tolerance patients, all eight studies found significant association between the two parameters, hereof five that found significant association between IENFD and all tested QST parameters. At the same time, only 21% of studies of non-DN etiology found the same association. These findings indicate that the etiology may be an important factor when examining the role of the IENFs of sensory dysfunction or pain, albeit other mechanisms play an important role as well, for example, the fact that QST is a cognitive component and central mechanisms of sensitization also likely play a role in nociception.
Other morphological changes
The estimation of intraepidermal nerve fiber density can be considered being relatively crude and simple measure that only takes into account the number of nerve fibers penetrating the epidermal–dermal boarder. Several studies have quantified other morphological changes in DN, including epidermal and dermal nerve fiber length density (NFLD) using stereology that may improve the diagnostic accuracy , quantification of nerve fibers in different skin layers that are effected in DN , and branching density that correlates with severity of DN . Another morphological change that has been studied is axonal swellings—enlargements that are visible on some of the nerve fibers, and are typically expressed as a swelling ratio (number of swellings per IENFD) . Axonal swellings are believed to represent degenerative changes on the nerve fibers and have been shown to contain watery axoplasm, neurofilaments, and abnormal mitochondria . Many studies have shown that axonal swelling ratio is higher in patients with neuropathy compared with those without neuropathy , including patients with DN . The clinical significance of these swellings is unclear, as they have both been associated with pain and to have no association with pain . A recent study including a total of 249 participants looked further into the association between axonal swellings and type 2 diabetes, neuropathy and pain. The study found that the swellings were related to type 2 diabetes rather than neuropathy or pain, and postulated that the swellings may be an early indicator of a disease affecting the small nerve fibers and may predict future nerve fiber loss . Analysis of axonal swelling ratio may even increase the diagnostic performance of skin biopsies .
Alternative markers used on skin biopsies
One of the advantages that skin biopsies have to offer is the amount of additional information that can be extracted from them. It is possible to use virtually any specific, reliable, and relevant antibody on them to get quantitative and morphological information about different subpopulations of nerve fibers as well as other cells and structures that are in the skin. A few examples of these analyses will be discussed below.
Biomarkers of pain pathophysiology
As already discussed, there is a weak association between IENFD and presence of pain or pain severity. It is, therefore, of interest to identify “biomarkers of pain pathophysiology,” or a marker that can readily distinguish between painful and nonpainful DN that would hold a potential to be a novel treatment target for painful DN. The nociceptors in the skin can be divided into two groups according to peptide expression. They are either peptidergic fibers that release calcitonin gene-related peptide or substance P, or they are nonpeptidergic fibers expressing receptor tyrosine kinase . Recent studies have shown that patients with painful DN have increased peptidergic fiber density compared with healthy participants without diabetes and diabetes patients without neuropathy . Furthermore, the peptidergic correlated with pain intensity while the IENFD did not. Similarly, growth-associated protein 43, a marker of regenerating nerve sprouts, has been shown to be higher in patients who describe their pain as burning .
Biomarkers identifying other structures than nerve fibers may also be of interest. Immune cells such as macrophages have been shown to be increased in the skin of patients with painful neuropathies because of diabetes and chemotherapy, compared with patients without pain and healthy controls . Another interesting pain-related structure that can be quantified using skin biopsies is the “nociceptive” Schwann cell. In a recent publication in science, an until-now-unknown organ likely playing an essential physiological role in sensing painful stimuli was identified in animal model of pain . The paper describes specialized cutaneous Schwann cells that initiate pain sensation and surround themselves around the nerves in the skin and feed them with nociceptive information. A recent paper by the same group followed up on the initial work and showed that these cells are also present in the human skin . However, they still remain to be quantified and compared between patients with and without painful DN and it is unclear what role they play in the human nociception. Taken together, these markers show promise and may represent novel treatment targets for painful DN.
Several other markers have been studied and compared between patients with and without DN or painful DN, including the TRPV1 that is responsible for heat sensation greater than 43°C , growth-associated protein 43 (GAP-43) that is believed to be a marker of regenerating nerve sprouts and has been associated with burning pain sensation , and Langerhans cells that also have been implicated with painful symptoms in painful DN .
Autonomic structures
A subset of DN patients has autonomic neuropathy and may have symptoms such as reduced sweat function. The skin biopsies also allow for assessment of autonomic structures in the skin, such as vascular, sudomotor, vasomotor, and pilomotor nerve fibers. It is therefore possible to perform density analysis of specific subpopulations of autonomic nerve fibers, nerve fiber innervation in sweat glands and their density and morphology, and arrector pili muscle, responsible for hair erection upon contraction. The vast majority of sudomotor fibers (cholinergic fibers) can be visualized using the vasoactive intestinal peptide antibody while pilomotor fibers (e.g., arrector pili muscle, predominantly noradrenergic) can be visualized using dopamine beta hydroxylase antibody. Gibbons and colleagues have shown a significant decrease of sudomotor innervation in patients with diabetes compared with healthy controls, including sweat gland nerve fiber density (SGNFD), and have compared different methods to quantify this measure . The study showed that there was a significant and relatively strong correlation between IENFD and SGNFD and that patients with more severe DN also had lower SGNFD. Although not studied in DN, detailed assessment of the autonomic innervation in skin biopsies taken from patients with Parkinson’s disease has shown abnormalities in autonomic nerves to vessels, sweat glands, and arrector pili muscle, and even Meissner corpuscles in glabrous skin . There is a need to further assess the autonomic structures in the skin in DN, and combining this analysis with IENFD estimation may even increase the specificity of IENFD in diagnosing DN.
Conclusion
Skin biopsies allow for a quantification of IENFD and assessment of nociceptive damage in DN. They are, however, not needed in certain clinical cases as they do not change the treatment plan. The skin biopsies represent an important research tool due to their ability to detect quantifiable molecular changes that increase our knowledge of why some, but not all, DN patients develop neuropathic pain and may even contribute to the development of mechanism-based treatment. Disadvantages of skin biopsies include that they require specialized and trained staff, they are invasive, and the quality of the analysis differs between laboratories and is dependent on staining quality.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree