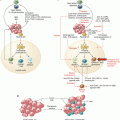
Fig. 33.1
Tissues of the body affected by immune-related adverse events
33.2 Incidence of Immune-Related Adverse Events
Among patients receiving ipilimumab, the most commonly reported adverse events include diarrhea, colitis, fatigue, pruritus, rash, and endocrinopathies [8], while for patients treated with PD-1 inhibitors, common adverse events include fatigue, rash, diarrhea, pruritus, arthralgia, and constipation [9–11]. The incidence of any grade irAE is reported to range from 15–90% [1, 12] in single-agent trials, while the rate of severe irAEs requiring immunosuppression and withdrawal of immunotherapy is estimated to be 0.5–13% [1, 3, 4, 13].
With combined anti-CTLA-4 and anti-PD-1 therapy, grade 3–4 drug-related adverse events occurred in 54% of patients, with the most common being colitis (17%), diarrhea (11%), and elevated alanine aminotransferase levels (11%). Immunosuppressive medications for the management of irAEs, including topical agents for dermatologic adverse events, were used in a higher percentage of patients receiving combination compared with ipilimumab alone (89% vs 59%) [12, 14, 15].
The risk of severe grade adverse events increased from 7 to 25% with an increase in the dose of ipilimumab from 3 to 10 mg/kg, largely due to an increase in the number of episodes of diarrhea. However, this pattern was not observed when nivolumab dosing was increased from 0.3 to 10 mg/kg. Similarly, occurrence of severe grade toxicities with pembrolizumab did not significantly increase with dose, occurring in 20% of patients treated with 2 mg/kg and 25% of patients treated with 10 mg/kg [16, 17]. As such, it appears that toxicities due to anti-CTLA-4 antibodies are dose dependent, whereas toxicities with anti-PD-1/PD-L1 antibodies are independent of dose.
33.3 Timing of Immune-Related Adverse Event Onset
The onset and outcome of irAEs with ipilimumab seem to vary according to the organs involved, and although most occur within the first 3 months of treatment, some specific toxicities are reported months after completion of therapy. The majority of toxicities appear temporally, with skin manifestations the earliest to appear at 2–3 weeks after the first dose of ipilimumab. Immune-mediated colitis and hepatitis appear at approximately 5–10 and 12–16 weeks, after the second and third doses, respectively. Endocrine dysfunctions present from the ninth week onwards following the fourth dose [1] and can take time to resolve or may even be irreversible, as is the case for most occurrences of hypophysitis. Immune-mediated pneumonitis is seen 8–14 weeks after treatment initiation [1], and immune-mediated nephritis appears later, after 14–42 weeks on immunotherapy [18].
33.4 General Considerations
Guidelines for the management of irAEs associated with ipilimumab were approved by the FDA as part of a risk evaluation and mitigation strategy. Anti-PD-1 antibodies, in particular nivolumab and pembrolizumab, seem to be better tolerated than ipilimumab, but adverse events related to these drugs require the same types of treatment. The guidelines provide a specific toxicity approach, depending on the organ or system involved but, in general, involve cessation of immunotherapy and initiation of steroid therapy for immunosuppression, with dosages and timing depending on the severity and type of toxicity.
For grade 1 toxicity, only symptomatic therapy should be used. For grade 2 toxicity, immunotherapy should be interrupted and treatment with oral steroids (prednisone 0.5–1 mg/kg/day or equivalent) should be started within 1 week after onset of symptoms if these persist. Immunotherapy may be resumed only if symptoms and signs of toxicity fall to grade 1 or resolve completely. For grade 3–4 toxicities, high doses of glucocorticoids (prednisone 1–2 mg/kg/day or equivalent) should be given. Doses should be gradually tapered when symptoms subside to grade 1 or less. Treatment with immune checkpoint inhibitors should be permanently discontinued. Finally, in cases of severe toxicity not responsive to high-dose corticosteroid therapy, administration of infliximab (5 mg/kg), a chimeric monoclonal tumor necrosis factor (TNF)-α inhibitor, should be considered. This may be repeated about 2 weeks after the first dose if symptoms fail to resolve. The use of an anti-TNF agent is based on its use in autoimmune diseases such as Crohn’s disease and ulcerative colitis. Histological studies of ipilimumab-induced immune-related colitis demonstrate the colonic mucosa infiltrated with both lymphocytes and neutrophils, similar to idiopathic ulcerative colitis. The early use of these agents allows for a quick resolution of adverse events and a reduction in the use of corticosteroids [19].
The use of corticosteroids for the treatment of irAEs does not seem to affect the efficacy of checkpoint inhibitors. Data derived from a large case series of patients (n = 298) receiving treatment with ipilimumab (3 mg/kg) at the Memorial Sloan Kettering Cancer Center outwith of clinical trials between April 2011 and July 2013 report the use of corticosteroids for the treatment of irAEs in 103 patients (35%) and treatment with anti-TNF in 29 patients (10%) who did not respond promptly to steroid therapy [20]. The OS and time to treatment failure were not affected by treatment with immunosuppressive therapy. There are less data on the effect of immunosuppressive agents on the efficacy of anti-PD-1 treatment, but based on what is currently known, there does not seem to be a reduction in the efficacy of anti-PD-1 therapy due to the use of immunosuppressive therapy for irAEs [21].
The association between irAEs and the efficacy of immune checkpoint inhibitors is controversial [22]. Data from early clinical trials of ipilimumab showed an association between increased irAEs and greater clinical benefit [23]. A pooled analysis of three phase II studies reported that patients with no or grade 1 irAEs achieved a disease control rate (DCR) of 20–24%, while a DCR of 34–43% was observed in patient with irAEs of at least grade 2. The OS in the two groups of patients was 8.2 months compared with 14.8 months [24]. In addition, in two trials of adjuvant ipilimumab in patients with high-risk melanoma, a significant association was found between relapse-free survival and irAEs [25]. In contrast, a retrospective study of 135 non-melanoma patients who received anti-PD-1 treatment found no significant correlation between ORR or OS and incidence of any irAE (p = 0.21) or the use of steroids (p = 0.27) [26]. The mechanism of action of infliximab is to block the ability of TNF to recruit neutrophils to the site of inflammation, which is unlikely to affect the antitumoral activity of immune checkpoint inhibitors.
33.5 Organ-Specific Immune-Related Adverse Events
33.5.1 Cutaneous Toxicity
Skin manifestations including rash/pruritus and mucositis are the most frequent irAEs associated with immune checkpoint inhibitors. Approximately 47–68% of patients treated with anti-CTLA-4 antibodies [23] and 30–40% of patients treated with anti-PD-1/anti-PD-L1 antibodies [27] experience skin toxicities of any grade.
The typical rash is reticular, maculopapular, and slightly erythematosus, especially developed on the trunk and extremities, and may be pruritic (Fig. 33.2). The majority of immuno-related rashes can be treated with topical creams based on corticosteroids. Itching, if persistent, can be treated with oral antihistamines. Grade 2 rash, especially if pre-existing, requires treatment with oral steroids. Grade 3–4 requires discontinuation of treatment and therapy with intravenous (IV) corticosteroids. A consultation with a dermatologist is recommended, as is a skin biopsy if possible.
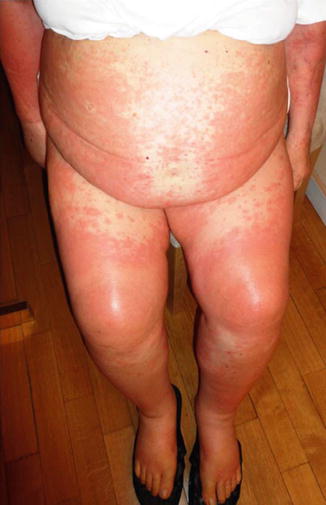

Fig. 33.2
Cutaneous rash in a patient treated with ipilimumab
Stevens-Johnson syndrome is a fairly rare occurrence that can be fatal (Fig. 33.3). In these patients, IV treatment with corticosteroids is always recommended. If there is no improvement in symptoms, the use of immunosuppressive agents in combination with corticosteroids should be considered [28]. Vitiligo is a common manifestation and generally occurs late following treatment with immune checkpoint inhibitors. It is characterized by a perivascular lymphocytic infiltrate in the deep dermis layer in proximity to melanocytes and is considered as a marker of response to treatment [29]. Oral mucositis is more common with anti-PD1 antibodies than with ipilimumab and usually requires treatment with topical corticosteroids and lidocaine, to be used after excluding the presence of candidiasis (Fig. 33.4).
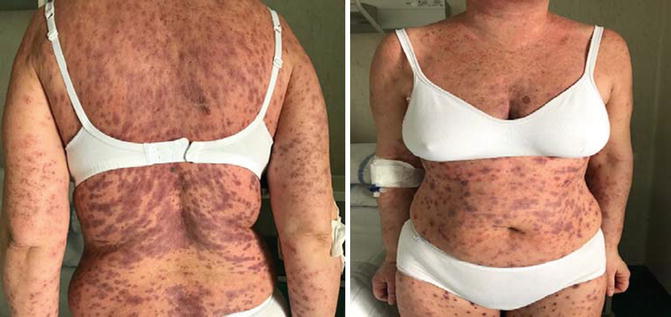
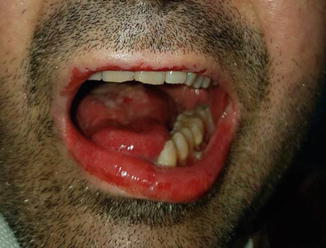

Fig. 33.3
Stevens-Johnson syndrome

Fig. 33.4
Mucositis induced by anti-PD-1 treatment
33.5.2 Gastrointestinal Toxicity
Diarrhea is one of the most common toxicities in patients receiving immunotherapy and must be diagnosed and treated in an adequate and timely manner to prevent it from developing into a serious adverse event. Generally, diarrhea appears after about 6 weeks of treatment [13] and is more often related to treatment with anti-CTLA-4 than with anti-PD-1 agents. Diarrhea has been reported in about 30% of patients with melanoma treated with ipilimumab, being grade 3–4 in less than 10% [30]. In a phase II study, the rate of severe diarrhea was higher in patients treated with ipilimumab 10 mg/kg than with 3 mg/kg (10% vs 1%) [31]. Similarly, in a phase III trial comparing ipilimumab 3 mg/kg and 10 mg/kg in patients with metastatic melanoma, GI toxicity was higher with the 10 mg/kg dose, with diarrhea reported in 79% of patients treated with 10 mg/kg versus 64% of patients in the 3 mg/kg arm. Grade 3–4 diarrhea was almost twice as common with ipilimumab 10 mg/kg compared with 3 mg/kg (34% vs 18%) [32]. Grade 3–4 diarrhea only occurs in 1–2% of patients treated with anti-PD-1 agents. GI toxicity is the main irAE with the combination of nivolumab plus ipilimumab, with an incidence of about 40%, including 9% of patients experiencing severe diarrhea and colitis [9, 10].
Enteritis, in some cases without diarrhea, can cause obstruction of the small intestine [33]. On biopsy, histology shows an edematous mucosa with rich infiltrate of neutrophils and/or lymphocytes [29, 30]. To date, there are no effective treatments to prevent the development of diarrhea and/or colitis. The use of budesonide to treat enteritis has been assessed but did not show any significant benefit [34].
In cases of diarrhea, the right level of hydration is critical, and the presence of other possible causes of diarrhea (e.g., Clostridium difficile infection) must be excluded. For grade 1 diarrhea, symptomatic treatment with loperamide is usually sufficient. For grade 2 diarrhea, suspension of immunotherapy and treatment with oral corticosteroids (e.g., prednisone 0.5–1 mg/kg/day or equivalent) are indicated. Treatment can be resumed in cases which resolve, but cases which do not resolve within 3–5 days should be treated similar to grade 3–4 events, which involves high-dose IV corticosteroids (e.g., methylprednisolone 1–2 mg/kg/day) and prophylactic antibiotic therapy. Treatment with anti-CTLA-4 and/or anti-PD-1 should be permanently discontinued. The patient should be clinically monitored because of the high risk for bowel perforation. In patients not responding to treatment with high-dose corticosteroids after about 3–5 days, treatment with infliximab 5 mg/kg is recommended and should be repeated after 2 weeks if incomplete resolution of symptoms.
33.5.3 Endocrine Toxicity
About 10% of patients treated with anti-CTLA-4 experience clinically significant endocrinopathy [35], while the incidence of endocrine disorders in patients treated with nivolumab is 14%, with 2% of events grade 3–4 in severity [4]. Less than 5% of patients experienced grade 3–4 endocrine toxicity with combination treatment [32]. The main types of endocrine toxicity derive from inflammation of the thyroid, pituitary, or adrenal glands and are often difficult to identify because they typically present with non-specific symptoms, such as fatigue, nausea, headache, and visual changes.
33.5.3.1 Thyroid Toxicity
Thyroid toxicity may involve both hypothyroidism and hyperthyroidism. In patients treated with anti-PD-1, the main toxicity is hyperthyroidism (high values of free thyroid fractions associated with suppressed thyroid-stimulating hormone [TSH]) which frequently occurs with mild symptoms and is diagnosed based on laboratory test results. After subacute onset, the disease can often evolve into Hashimoto’s thyroiditis with hypothyroidism. In patients treated with anti-CTLA-4s, the onset of Hashimoto’s thyroiditis without preceding hyperthyroidism is more frequent. The disease typically has a slow progression with gradual onset of symptoms; however, cases of acute onset with myxedema crisis have been reported [36]. Laboratory results show increased values of TSH, free thyroid-reducing fractions, and thyroid autoimmunity (antithyroid peroxidase positivity of autoantibodies and anti-thyroglobulin). Treatment is based on the use of levothyroxine (L-thyroxine), as a thyroid hormone replacement.
33.5.3.2 Hypophysitis
Hypophysistis has been reported to occur with different frequencies, ranging from 0–25% (average 4%), and is most commonly encountered in men. It occurs almost exclusively in patients treated with anti-CTLA-4 rather than anti-PD-1, possibly because of different distributions of CTLA-4 and PD-1 in the tissue. It has been hypothesized that anti-CTLA-4 antibodies may cause pituitary toxicity if bound to CTLA-4 antigen expressed “ectopically” on pituitary endocrine cells. Typically, it occurs after 6–8 weeks of treatment. The initial symptoms often consist of fatigue and hypotension and in overt forms can present as mass effect-related symptoms (e.g., headache, decreased visual acuity, diplopia, etc.) associated with failure of the pituitary axes (hypocortisolism, hypothyroidism, hypogonadism, panhypopituitarism).
Magnetic resonance imaging (MRI) may show a pituitary gland uniformly increased in volume with intense and homogeneous contrast enhancement. A very serious complication is the advent of an adrenal crisis which can lead to serious negative outcomes including death if not promptly recognized and controlled. Even in these cases, symptoms can be non-specific, e.g., fatigue, asthenia, anorexia, weight loss, nausea, vomiting, and hypotension. Laboratory tests typically show a reduction in circulating cortisol and adrenocorticotropic hormone (ACTH) values and reduced daily urinary excretion of cortisol, often associated with reductions in follicle-stimulating hormone (FSH), luteinizing hormone (LH), and TSH, with central hypothyroidism.
High doses of glucocorticoids are reserved for patients who have serious mass effect-related symptoms, such as severe headache, visual field disturbance, or simultaneously present with other irAEs. Physiological replacement doses should be considered for patients with corticotropic deficiency since pharmacological glucocorticoid therapy is not clearly associated with improved outcomes in such patients [37]. In case of grade 3–4 toxicity, treatment should be discontinuated.
Type I diabetes has been highlighted with both anti-PD-1 and anti-CTLA-4, with an incidence of 8% in patients treated with combination therapy. Treatment with immune checkpoint inhibitors should be deferred in patients with grade 3 hyperglycemia, while treatment should be permanently discontinued with grade 4 toxicity. Corticosteroids are not indicated.
33.5.4 Hepatic Toxicity
Liver toxicity typically occurs in less than 10% of patients [29]. Among patients receiving ipilimumab 3 mg/kg, severe, life-threatening, or fatal hepatotoxicity occurred in 2%, while the incidence of all grade hepatitis was 2.3% with nivolumab monotherapy. However, occurrence was higher (13%) in patients treated with ipilimumab plus nivolumab in combination. Very often, patients are asymptomatic and the only indicator of toxicity is increased transaminases. A worsening of total bilirubin is less often found and usually occurs late. Generally, liver toxicity begins after about 8–12 weeks of treatment.
Although clinical trials have excluded patients with a history of hepatitis B and C, these patients have been treated in clinical practice. Although data are limited, the presence of previous viral hepatitis does not seem to increase the risk of hepatotoxicity [38, 39]. Before starting treatment with immune checkpoint inhibitors, it is mandatory to assess liver function and markers for hepatitis B or C virus. In all patients with HBsAg positivity, early antiviral treatment may be needed in high viral load (HBV DNA).
In patients with grade 1 toxicity (i.e., asymptomatic increases in transaminases and hyperbilirubinemia), treatment can be continued, and liver function tests should be monitored until resolution. Grade 2 events require the interruption of immunotherapy until resolution. Oral prednisone 1 mg/kg/day should be administered. In patients with grade 3–4 events, treatment should be permanently discontinued; high-dose IV glucocorticoids (methylprednisolone 2 mg/kg/day) are recommended. Mycophenolate 500 mg every 12 h should be considered if liver enzymes are still elevated after 48 h of treatment. If no improvement occurs in the following 5–7 days, tacrolimus at the dosage of 0.10–0.15 mg/kg/day is recommended. Infliximab is not recommended because of its hepatotoxicity.
33.5.5 Pulmonary Toxicity
Grade 3 or higher pneumonitis has been reported in 5–7% of patients with NSCLC treated with nivolumab or pembrolizumab [40]. However, the incidence of symptomatic pneumonitis is only 1% with ipilimumab [41]. About 7% of patients treated with nivolumab plus ipilimumab had pneumonitis, with only 1% of grade 3–4 severity [32]. A recent systematic review and meta-analysis suggest that the overall incidence of pneumonitis during PD-1 inhibitor monotherapy was 2.7% (95% CI: 1.9–3.6%) for all grades and 0.8% (95% CI: 0.4–1.2%) for grade 3 or higher pneumonitis. The incidence was higher in patients with NSCLC compared with melanoma for pneumonitis of all grades (4.1% vs 1.6%; p = 0.002) and grade 3 or higher (1.8% vs 0.2%; p < 0.001). The incidence of pneumonitis was more frequent during combination therapy than monotherapy for all grades (6.6% vs 1.6%; p < 0.001) and grade 3 or higher (1.5% vs 0.2%; p = 0.001) pneumonitis [42, 43]. Several factors, such as pre-existing lung damage due to tumor burden, smoking, chronic obstructive pulmonary disease, pulmonary fibrosis, and variable expression of PD-L1 on normal lung tissues, may play a role in this although the exact cause of this difference is still unknown.
Pulmonary toxicity should be considered whenever patients present with symptoms of a respiratory infection, new-onset cough, or wheezing. In symptomatic patients, a chest CT scan and a pulmonary consultation are recommended, as is initiation of oral or IV corticosteroids. In patients with moderate or severe symptoms, a diagnostic bronchoscopy that can assess whether there is widespread lymphocytic infiltration is advised. If the toxicity is grade 1 with only asymptomatic radiological signs, treatment suspension of 2–4 weeks until resolution of radiographic findings should be considered. If grade 2, suspension of treatment is mandatory and treatment with oral corticosteroids should be initiated. In severe or recurrent cases, IV treatment with methylprednisolone 2 mg/kg or equivalent may be appropriate, and immune treatment should be permanently discontinued.
Although rare, there have been documented cases of pulmonary sarcoidosis in patients treated with immune checkpoint inhibitors [44].
33.5.6 Renal Toxicity
Renal failure has been reported in patients treated with ipilimumab, anti-PD-1s, and combination therapy. Immune checkpoint inhibitors can cause acute kidney injury that presents similar to other drug-induced tubulointerstitial nephritis. Cortazar et al. analyzed data from published phase II and III trials enrolling 3695 patients and reported an overall incidence of acute kidney failure of 2.2%, with 0.6% grade 3–4 events. The median duration until the appearance of kidney injury was 13 weeks [45]. Acute kidney failure occurred more frequently in patients treated with nivolumab plus ipilimumab (4.9%) than in patients treated with anti-PD-1 monotherapy. The most common signal of acute kidney failure was an increase in serum creatinine, which occurred in all affected patients. Pyuria (68%) and hematuria (16%) were also frequently noted in patients with renal impairment. In patients with suspected immune-related renal failure, a renal consultation should be sought early and a biopsy performed if possible. In patients with grade 2–3 renal toxicity, treatment should be suspended and steroids are recommended. Treatment can be resumed if symptoms improve to grade 1 severity. High doses of corticosteroids and treatment discontinuation are recommended for grade 4 toxicity.
33.5.7 Neurological Toxicity
Based on data in prospective studies, the overall incidence of any grade of neurotoxicity is 3.8% with anti-CTLA-4 antibodies, 6.1% with anti-PD-1 antibodies, and 12.0% with combination therapy [46]. However, most of these neurological adverse events are grades 1–2 and consist of non-specific symptoms such as headache (55%), dysgeusia (13%), or dizziness (10%). The incidence of grade 3–4 events was 1% across all treatments. On the basis of the nervous system area involved, neurotoxicity can be classified as encephalopathy, myelopathy, pure meningitis, meningoradiculitis, Guillain-Barre-like syndrome, peripheral neuropathy, or myasthenic syndrome. The spectrum of neurological symptoms appears to be highly heterogeneous, including headache, fever, tiredness or weakness, confusion, memory problems, sleepiness, hallucinations, seizures, and stiff neck. In the literature, several cases of serious toxicity have been reported, such as reversible posterior encephalitis syndrome [47], Guillain-Barre syndrome [48], myasthenia gravis, transverse myelitis [49], and demyelinating polyneuropathy [50].
All serious neurological irAEs should be treated with high-dose corticosteroids, and a neurologist should be consulted for differential diagnosis and additional therapy.
33.5.8 Rheumatological Toxicity
Arthralgia and arthritis are the most commonly reported rheumatic and musculoskeletal irAEs associated with immune checkpoint treatment. The incidence of arthralgia secondary to nivolumab in phase III trials ranges from 5 to 16% [51], and similar rates have been reported with ipilimumab monotherapy [52]. With combination therapy, incidence of arthralgia was about 10% [32]. Thus, although arthritis is a very common manifestation of autoimmune disease, it has not been reported as frequently as an adverse event in clinical trials of checkpoint inhibitors as might have been expected. One reason for this could be that there are several mutually exclusive ways to code musculoskeletal adverse events in the current system. For example, arthralgia, arthritis, joint effusion, and musculoskeletal pain are all potential options for coding the same event. Furthermore, its incidence could be underrepresented because most clinical trials report only grade 3–4 toxicity and arthritis is often considered to be less severe than other adverse events [53]. No observational studies that monitored patients for inflammatory arthritis with confirmation by a rheumatologist have been reported. However, in a case series of nine patients treated with immune checkpoint inhibitors and who developed inflammatory arthritis, clinical presentation was variable and involved large and small joints, with or without systemic involvement (colitis, urethritis) and autoantibody detection. Patients were treated with corticosteroids and some also required methotrexate and/or anti-TNF treatment [54]. The same series also reported on four patients who developed sicca syndrome while receiving checkpoint inhibition therapy. Symptoms of dry mouth were more severe than dry eyes in all four patients. Three had positive anti-nuclear antibodies. One patient was positive for La/SS-B antibodies and had parotitis treated with 6 weeks of prednisone that resulted in complete resolution of symptoms. All patients were negative for Ro antibodies [54].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree