Introduction
Sex steroids are fundamentally important to human sexual differentiation and maturation. Given the anatomical differences evident between the human male and female phenotype, it is thus no surprise that sex steroids have wide-ranging effects upon the body’s structural tissues, including bone, muscle, and fat.
This chapter will consider postpubertal sex steroid production, peripheral effects, and changes across the life course, with a focus on how these changes affect structural tissues. The role of sex steroids in sexual maturation and prepubertal growth is beyond the scope of this text.
Sex steroid biology
The vast majority of sex steroids are produced in two main locations: the zona reticularis of the adrenal glands and the gonads; the testes in the male and ovaries in the female. The contribution of each of these varies over the life course. Sex steroids can be broadly categorized into three groups. The androgens are typically considered “male sex steroids,” while the estrogens and progestogens are considered “female sex steroids.” While the adrenal gland produces primarily androgens and a small quantity of progestogens, the gonads produce hormones from all three categories.
Adrenal sex steroid production
In both males and females the adrenal glands predominantly produce the androgen dehydroepiandrosterone-sulfate (DHEAS). As with many endocrine hormones, its production is under negative feedback control by a tripartite loop known as the hypothalamic-pituitary-adrenal axis. The relevant hormones and tissues in this pathway are as follows:
- ●
Hypothalamic paraventricular nucleus—Corticotropin-releasing hormone (CRH)
- ●
Anterior pituitary corticotrope cells—Adrenocorticotropic hormone (ACTH)
- ●
Adrenal cortex zona reticularis—Dehydroepiandrosterone-sulfate (DHEAS)
CRH stimulates ACTH, which, in turn, stimulates the adrenal cortex to produce both DHEAS and cortisol, a glucocorticoid. While DHEAS exerts no negative feedback on CRH or ACTH, this feedback is exerted by cortisol, thus completing a feedback loop that regulates DHEAS concentrations.
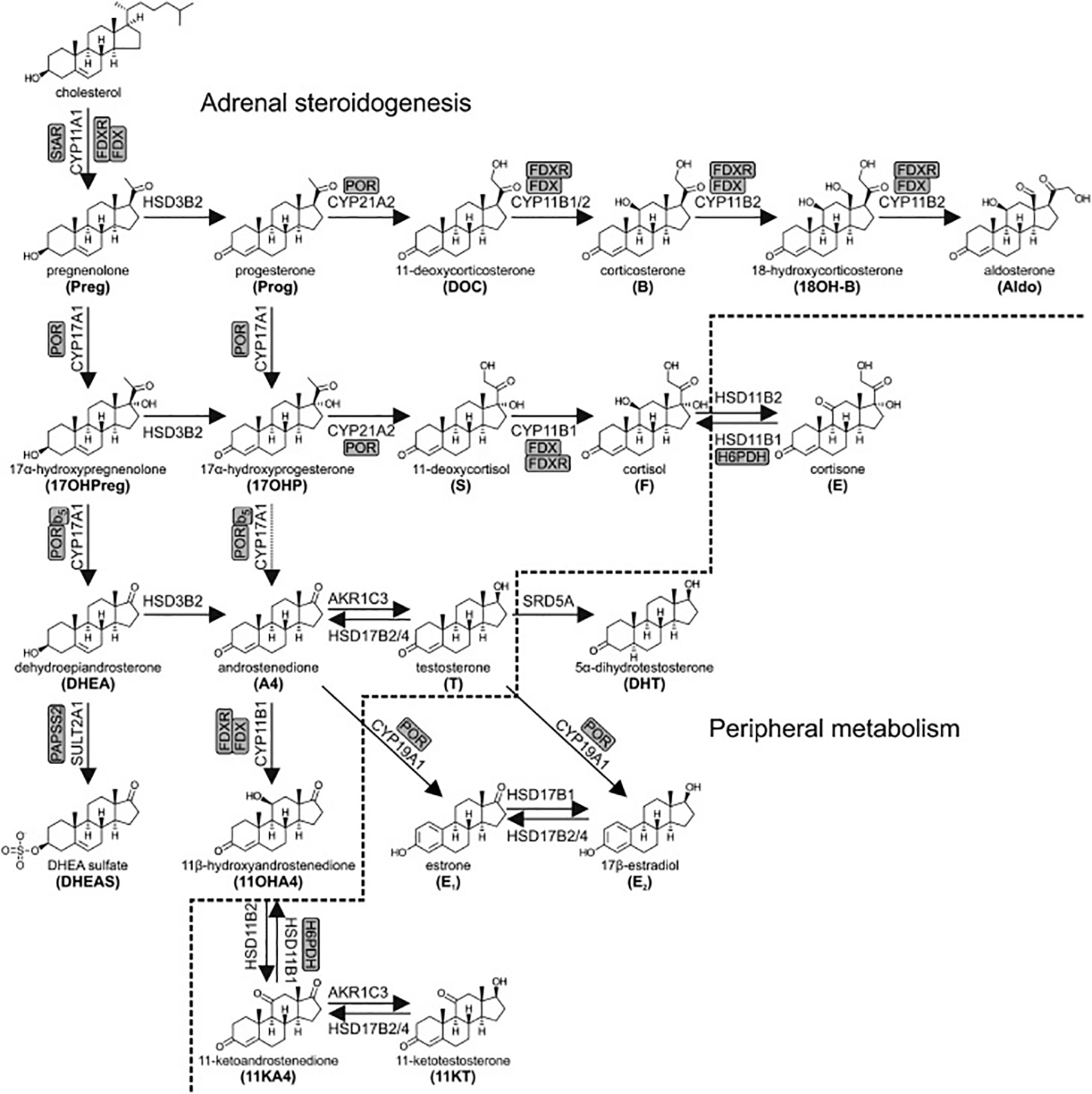
In addition to DHEAS, smaller concentrations of other weak androgens such as androstenedione and progestogens are also produced by the adrenal gland. Their production is similarly under the control of ACTH and ultimately CRH ( Fig. 1 ).
Changes across the lifespan
Adrenal androgen production does not commence until the period of development known as adrenarche, which usually occurs between 6 and 10 years of age. Adrenal androgen production increases during puberty to reach a peak during young adulthood. Levels then reduce due to a process is known as adrenopause, which commences between 30 and 40 years of age. This continues until approximately the age of 70 when DHEAS levels are 5%–10% of the young adult peak .
Gonadal sex steroid production
In males, the predominant sex steroid-producing organs are the testes, while in females this role is fulfilled by the ovaries. As is the case in the adrenal cortex, gonadal sex steroid production is under negative feedback control. This feedback loop is known as the hypothalamic-pituitary-gonadal axis. While the upper two elements of this loop are conserved between the sexes, the gonadal elements differ. The relevant hormones and tissues in this pathway are as follows:
- ●
Hypothalamic median eminence—Gonadotropin-releasing hormone (GnRH)
- ●
Anterior pituitary gonadotroph cells—Luteinizing hormone (LH) and follicle-stimulating hormone (FSH)
Males
- ●
Testicular Leydig cells—Testosterone
Females
- ●
Ovarian theca cells—Progesterone and testosterone
- ●
Ovarian granulosa cells—Estradiol
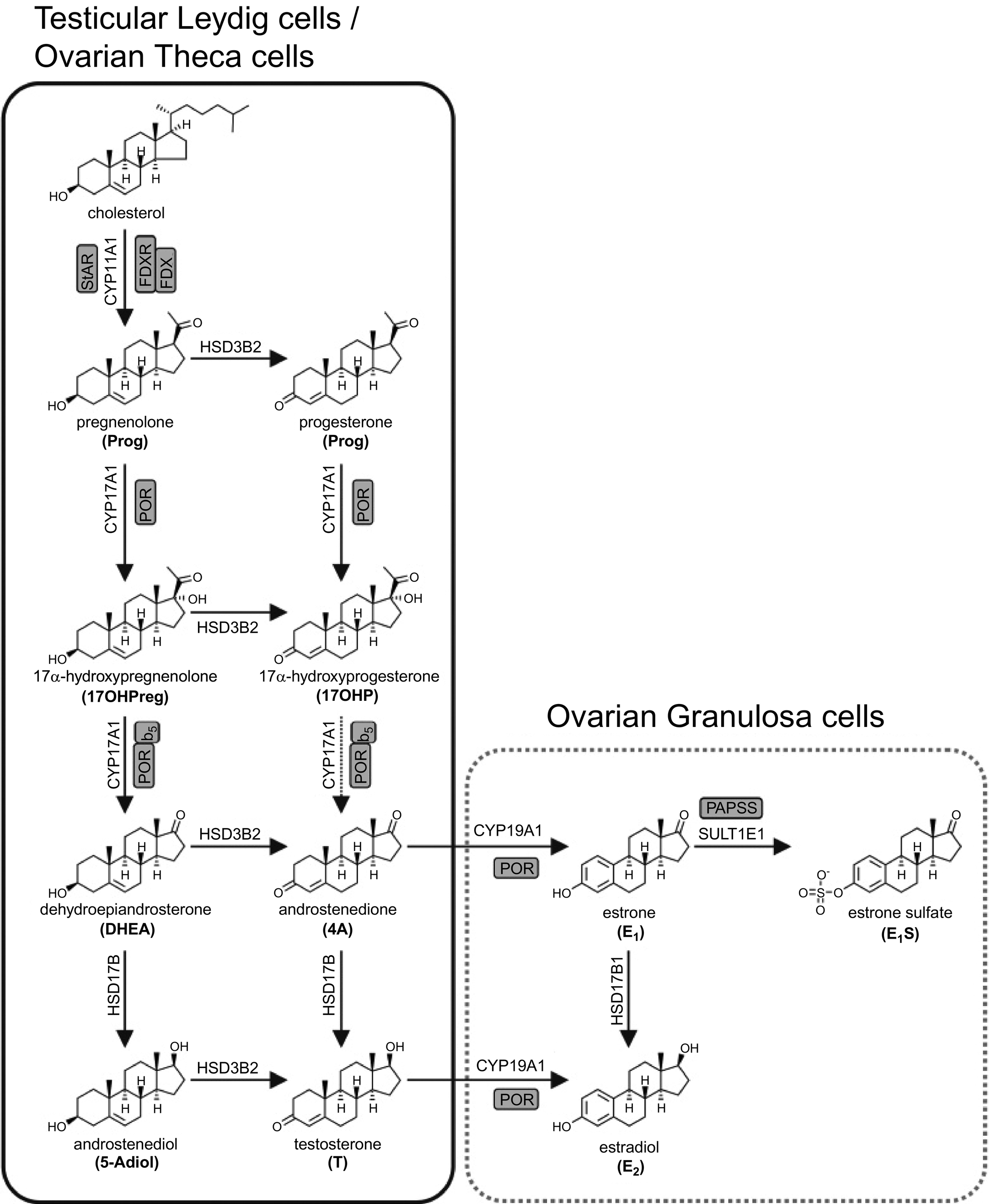
GnRH stimulates LH and FSH. LH stimulates the male testicular Leydig cells and female ovarian theca cells to produce testosterone in both and additionally progesterone in the case of theca cells. FSH stimulates the female ovarian granulosa cells to produce estradiol. In general, gonadal sex steroids exert negative feedback upon GnRH, LH, and FSH. However, the cyclical nature of the feedback loop in females who are menstruating is significantly more complex and such a discussion is beyond the scope of this text.
While testosterone is the major gonadal sex steroid produced in males, estradiol is also found in the male circulation and tissues. Only 20% of circulating estradiol is synthesized directly in the testes. The rest is produced by aromatization of androgens (predominantly testosterone but with some contribution from DHEAS) in bone, adipose, skin, brain, and other tissues, from where it is released back into the circulation ( Fig. 2 ).
Changes across the lifespan—Males
Following infancy, gonadal sex steroid production in males commences at the onset of puberty, LH causes adult Leydig cells to develop and begin producing testosterone. Gonadal sex steroids reach a peak at approximately 20 years of age in males with a gradual decline thereafter. This decline accelerates around the eighth decade of life and is occasionally termed andropause. However, it should be noted that there is disagreement over what constitutes physiologic vs pathologic reductions in androgen levels in males.
Changes across the lifespan—Females
Following infancy, gonadal sex steroid production in females commences at puberty when LH stimulates ovarian theca cells to produce both progesterone and testosterone, while FSH causes granulosa cells to convert thecal androgens to estradiol. Mean levels peak between 16 and 20 years of age and remain stable for the next three decades. Of course, levels of both estradiol and progesterone vary cyclically during the normal menstrual cycle, and change significantly over the course of pregnancy and for the duration of breastfeeding. When menopause occurs, usually between 50 and 55 years of age, there is a rapid and massive reduction in estradiol over no longer than a few years to levels similar to those found in males. Following menopause, most circulating estradiol in females derives from peripheral aromatization of adrenal androgens, primarily in adipose tissue.
Testosterone levels in females, as in males, peak around approximately 20 years of age before rapidly reducing over the next two decades, remaining stable thereafter. Female testosterone is produced exclusively by the ovarian theca cells.
Mechanism of action
In the circulation, steroid hormones are extensively bound to both specific (e.g., sex-hormone-binding globulin) and nonspecific (e.g., albumin) plasma proteins. As lipid-derived hormones, they readily diffuse across the lipid bilayers of cell membranes. However, membrane transporters for steroid hormones do exist and modulate the rate of entry into certain cells. Once inside the cytoplasm of steroid-responsive cells, steroid hormones bind to their specific receptor, activating it. This activated receptor-hormone complex is then able to translocate into the nucleus where it can bind to appropriate deoxyribonucleic acid (DNA) sequences, causing transcription of messenger ribonucleic acid (mRNA) to occur, which is in turn translated into proteins.
The androgen, estrogen, and progesterone receptors have two, two, and three known isoforms, respectively. It is the tissue-specific expression of these isoforms and their respective affinities for various sex steroids that determines some of the tissue-specific heterogeneity in sex steroid effects.
It is typical to differentiate between potent and weak forms of the three types of sex steroids, based on their affinity for their respective receptors. Estradiol is the only potent estrogen while progesterone is the only potent progestogen. Testosterone and its peripheral metabolite dihydrotestosterone (DHT) are both potent androgens.
Sex steroid effects on muscle
Androgens
Testosterone is the major sex steroid that influences skeletal muscle growth with the trophic effects of testosterone on muscle mass and myocyte protein synthesis well-established . Muscle mass is also increased via the growth hormone (GH)/insulin-like growth factor 1 (IGF-1) pathway, with the magnitude of testosterone’s effects comparable to this pathway. Testosterone levels correlate with muscle strength independent of muscle mass, suggesting trophic actions on muscle function in addition to tissue volume . While testosterone exerts direct effects upon myocyte-expressed androgen receptors, much of its effects are, in fact, exerted through its metabolite DHT. Myocytes express 5-alpha reductase (5AR), an enzyme that converts testosterone to the much more potent DHT in situ, which binds to androgen receptors with twice the affinity of testosterone while exhibiting a fivefold slower dissociation rate .
The direct and independent effects of DHEAS on muscle bulk are less clear with conflicting evidence from related lines of enquiry. While DHEAS levels have been found to correlate with muscle force per cross-sectional area in postmenopausal women in one study, a similar relationship was observed for other sex steroids in the same study . A relatively small, controlled cross-over trial found 6 months of Dehydroepiandrosterone (DHEA) supplementation (functionally similar to DHEAS) to be associated with increased muscle strength in males but not females . A more recent randomized controlled trial found 6 months of DHEA supplementation had no effect on muscle strength in males or females, but in a 4-month extension during which participants engaged in a weight-training program, muscle strength was increased compared with placebo .
It has been suggested that the main role of DHEAS in the musculature is to act as a precursor for testosterone and estradiol production since the relevant enzymes in the steroid biosynthesis pathway are expressed by muscle cells. Given the conflicting evidence around its effectiveness, a 2011 review found no benefit of DHEA replacement in postmenopausal women .
Estrogens
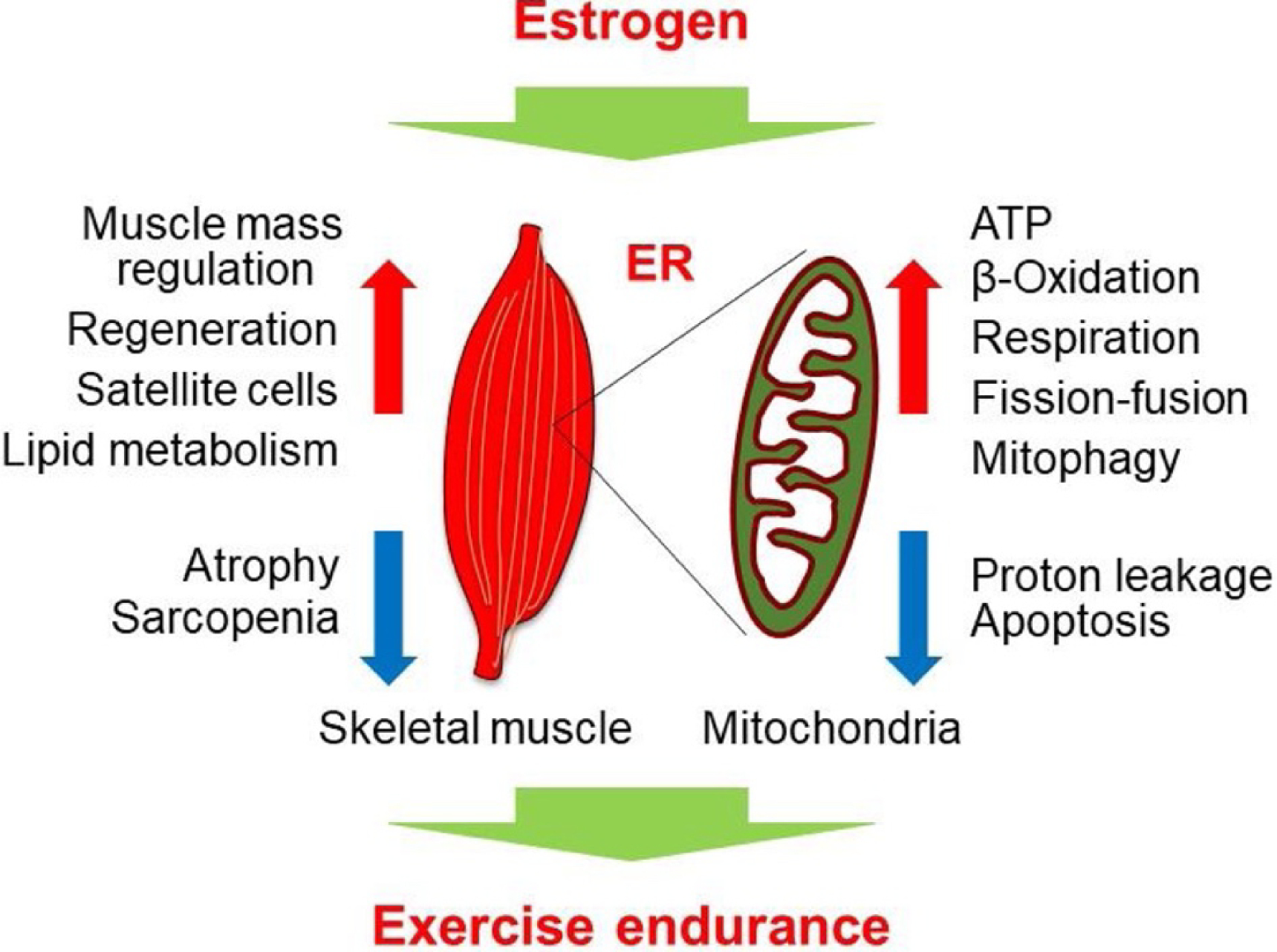
The evidence for a direct effect of estrogen on skeletal muscle is conflicting. While animal models suggest a clear role of estrogens in contributing to muscle growth, human studies of replacement differ in their conclusions. In a small, controlled cross-over trial, 3 months of hormone replacement therapy (HRT) given to early postmenopausal women resulted in an increase in total body lean mass, presumed to be muscle mass . However, in a similarly-sized prospective cohort trial, 64 weeks of subdermal estradiol implants given to postmenopausal women of similar ages to the previous trial did not result in any significant changes in appendicular skeletal muscle mass . There is some evidence that estrogens play different and at times opposing roles within the muscle via the alpha and beta isoforms of the estrogen receptor .
Estrogens may impact skeletal muscle indirectly through modifying levels of spontaneous activity and resting energy expenditure. It is plausible that declining estrogen levels may contribute to disuse atrophy of muscle through these indirect mechanisms ( Fig. 3 ).
Progestogens
The progesterone receptor is expressed in skeletal muscle cells and progesterone appears to have a direct trophic effect on muscle. In a study of replacing sex steroids in a menstrual cycle-mimicking manner in postmenopausal women, three simulated menstrual cycles of progesterone (three 14-day periods) resulted in increased muscle protein synthesis as measured by mRNA expression . It is unclear whether this translates to increased muscle mass or strength. Progesterone is not currently thought to play a significant role in skeletal muscle growth or strength, but perhaps plays a role in modifying the effects of estradiol on muscle in females.
Male physiology
The rapid increase in testosterone during male puberty is associated with marked gains in muscle mass, with considerable differences between males and females becoming evident over this period. As testosterone decreases following the young adult peak, so does muscle strength and functional performance. It is thought that testosterone explains much of the age-related decline in muscle strength seen in males. This relationship has been explored in numerous cohort studies including the Massachusetts Male Aging Study (muscle strength) and the Framingham Offspring Study (functional performance) . Further evidence for this are studies showing an improvement in functional performance with testosterone replacement in older males, most notably the Testosterone in Older Men with Mobility Limitations (TOM) trial, although cardiovascular-related adverse events were also increased .
However, the relationship between testosterone-related declines in muscle strength and impaired functional performance remains unclear, as does the clinical utility of testosterone supplementation in older males for this purpose . In one 20-week study of healthy men aged 60–75, testosterone supplementation resulted in an increase in both muscle mass and strength but not functional performance . It is also possible that testosterone augments the effects of exercise. In the Testosterone Trials, daily testosterone supplementation for 12 months resulted in improved physical function in those participants with a baseline walk speed of at least 1.2 m/s but not in those with slower walk speeds, suggesting benefit only in those who were likely able to engage in more physical activity and exercise . A recent systematic review and metaanalysis of this question suggest that both testosterone supplementation and exercise have independent beneficial effects upon muscle strength while testosterone alone increases muscle mass . Additionally, the combination of exercise and testosterone supplementation appears to have a greater effect on both parameters than either intervention alone.
While male myocytes express the estrogen and progesterone receptors, the low levels of both of these hormones in circulation mean that serum-derived estrogens and progestogens have minimal effects upon the male musculature. There is also reduced expression of aromatase in muscle compared with other tissues, meaning that there are only low levels of in situ production of estrogen from circulating androgens . What is ultimately clear, however, is that while testosterone levels are related to muscle strength in older males, estradiol is not .
Female physiology
As in males, female muscle mass increases during puberty though to a lesser degree, likely explained by the differential testosterone and estrogen levels and effects on muscle. It has long been observed that there is a general decline in female muscle mass from around 30 years of age, which accelerates at the menopausal transition around the age of 50 . It is likely that this acceleration from menopause is related to the precipitous decline in estradiol levels at this time. This is likely largely mediated via a loss of estrogens role in muscle repair leading to accumulation of damage and subsequent atrophy . Androgens likely play a role in the determination of female muscle mass, however, the fact that androgen levels are gradually declining prior to menopause with no precipitous decline at this time (DHEAS often increases around the menopause before continuing its age-related decline ), suggests a significant role for estrogens in determining female muscle mass.
Progestogens are thought to play a limited role in determining female muscle strength or mass and are implicated in cyclical changes in muscle protein synthesis over the menstrual cycle as well as during pregnancy and breastfeeding . However, these changes appear not to affect muscle contractile characteristics . The role of progesterone in age-related skeletal muscle changes remains minimally investigated.
Sex steroid effects on bone
Androgens
Androgens appear to have markedly reduced direct effects upon bone compared with estrogens. In both males and females, the androgen receptor does not appear to have a direct regulatory effect upon osteoclasts . However through aromatization of testosterone to estradiol locally within the bone itself, serum testosterone levels do have downstream effects upon bone metabolism, mediated via estradiol. There is some evidence that testosterone has an estradiol-independent role during skeletal growth and maturation preadulthood. Some of this action is mediated via the GH/IGF-1 pathway and appears to contribute to periosteal apposition, which has the net effect of increasing bone size . Testosterone also appears to have greater trophic effects upon cancellous rather than cortical bone. This may relate to differential expression of aromatase between these bone compartments, or an estrogen-independent mechanism.
While there is some evidence that DHEAS and other weak adrenal androgens improve bone mineral density (BMD), evidence is inconsistent . The role of DHEAS in bone metabolism is predominantly to provide a substrate for peripheral aromatization into estradiol. If there is a true independent effect, it is weak compared with estradiol and testosterone and likely does not play a significant role in mediating age-related changes.
Estrogens
In contrast to muscle, the predominant sex steroid influencing bone metabolism is estradiol. The activated estrogen receptor has direct effects upon both osteoblasts and osteoclasts, stimulating bone production by the former and reducing bone resorption by the latter. Preclinical and clinical evidence for this relationship is strong but perhaps nowhere more so than in the Women’s Health Initiative trial. In this seminal trial, hormone replacement therapy (HRT) prescribed to postmenopausal and thus estradiol-deficient women resulted in marked improvements in BMD compared with those receiving placebo .
Estrogen signaling appears to have greater effects upon cortical bone than cancellous bone, though it is integral to the development of both . This is likely mediated by differential expression of the two estrogen receptor isoforms, alpha, and beta, between the two bone compartments. While both are expressed in cancellous bone, it is predominantly the alpha isoform that is found in cortical bone. Since the beta isoform appears to antagonize the trophic effects of the alpha isoform in bone, this differential receptor expression likely explains these compartment-specific effects .
Progestogens
There is some evidence that progesterone may have bone-trophic effects . However, any effect is small compared with other sex steroids and progesterone does not play a role in determining age-related skeletal changes.
Male physiology
Male and female skeletal maturation across puberty is similar, with high levels of sex steroids during this period contributing to longitudinal bone growth, bone mass accrual, and eventually epiphyseal closure to determine the final adult height. Given the low levels of circulating estradiol in males, these effects are predominantly mediated by estradiol produced in situ within bone following aromatization of testosterone. The strongest evidence for this derives from a 3-week trial of pharmacologically suppressing both testosterone and estradiol production in healthy older males, in which the participants were randomized to replacement of none, either, or both of testosterone and estradiol . Comparing bone formation and resorption markers between the groups provided clear evidence that suppressing sex steroid production profoundly increases bone resorption while suppressing its formation. Replacing estradiol alone or both hormones reverses these negative effects. Whereas replacing testosterone, while the body’s ability to convert this to estradiol is pharmacologically suppressed, has little to no effect.
Testosterone’s estradiol-independent effects upon periosteal apposition and cancellous bone in preference to cortical bone appear to account for some of the gender differences in bone size that become established during puberty. These effects account for the observation that males typically have a greater bone cross-sectional area than females of an equivalent age .
The gradual reductions in circulating androgens that occur with aging account for much of the age-related decline in BMD. Some of the strongest evidence for this derives from the Testosterone Trials. In these trials of testosterone replacement for 12 months in males at least 65 years of age with low testosterone levels, an improvement in both volumetric BMD and estimated bone strength was observed in the testosterone-treated versus placebo group .
Female physiology
As the main circulating sex steroid in females and the predominant effector of bone metabolism within this group, the rapid increase in estradiol during female puberty accounts for much of the skeletal development and maturation during this time. The differential expression of estrogen receptor isoforms between the bone compartments results in a higher cortical: cancellous bone ratio than in males.
Subsequent to puberty there is a small amount of bone loss between early adulthood and menopause. Once the menopausal transition commences, however, there is rapid and marked bone loss, following which the rate of bone loss parallels that in similarly aged males. Postmenopausal female bone density is determined most strongly not by serum estradiol but by serum testosterone , consistent with the predominant source of estradiol in the postmenopausal state being local production from androgen aromatization.
In females, there is weak and inconsistent evidence of a direct effect of androgens on bone metabolism . The role of circulating androgens in female bone metabolism is predominantly to act as a substrate for peripheral conversion to estradiol. Strong evidence for this is presented by the accelerated bone lone caused by aromatase inhibitor used for the adjuvant treatment of postmenopausal female breast cancer . This is due to the postmenopausal state being characterized by minimal circulating estradiol such that bone health relies upon local production through aromatization of testosterone and DHEAS ( Fig. 4 ).