Gene mutation
Serous tumor of LMP
Low-grade serous carcinoma
References
TP53
0–8 %
0–8.3 %
KRAS
33–61.8 %
19–41 %
BRAF
14.5–28 %
2–33 %
Clinical Behavior and Treatment
Surgery is a major modality of treatment in low-grade serous carcinoma, as it is in all histologic subtypes. For most patients, primary surgery, including comprehensive surgical staging for patients with apparent early-stage disease and cytoreductive surgery for those with metastatic disease, is the initial treatment. Fertility-sparing surgery is an option for selected young patients. For selected women with extensive metastatic tumor or significant comorbidities, neoadjuvant chemotherapy with interval cytoreductive surgery may be recommended. In such cases, either fine needle aspiration/core biopsy or a minimally invasive surgical procedure to establish an accurate diagnosis is performed prior to starting chemotherapy.
Several predominant themes have emerged from studies of the clinical course of low-grade serous carcinoma of the ovary or peritoneum. In an ancillary study of Gynecologic Oncology Group (GOG) protocol 182, Fader et al. reported the details regarding 189 patients with FIGO grade 1 serous carcinoma (a surrogate for low-grade serous carcinoma) [96]. On multivariate analysis, only residual disease status following primary surgery was significantly associated with overall survival. Patients with microscopic residual had a significantly longer median progression-free (33.2 months) and overall survival (96.9 months) compared with those with residual 0.1–1.0 cm (14.7 months and 44.5 months, respectively) and more than 1.0 cm of residual disease (14.1 months and 42.0 months, respectively). The overall pattern of these results closely resembles that of epithelial ovarian cancer in general. In a second study from the same dataset, serum CA 125 values were analyzed [98]. Although pretreatment CA 125 was not prognostic of outcome, patients with CA 125 levels that normalized after one to three cycles of chemotherapy were 60–64 % less likely to experience disease progression as compared to those who never normalized or normalized after 4 cycles (p ≤ 0.024). Normalization of CA 125 levels before the second cycle was negatively associated with death, with an HR of 0.45 (p = 0.025).
Previs et al. reported the Duke experience with 81 women with low-grade serous carcinoma of the ovary [100]. On multivariate analysis, obesity (HR = 2.8) and optimal tumor debulking (HR = 0.05) were significant predictors of overall survival. Additionally obesity was not associated with worse disease-specific survival, suggesting that mortality of obese patients may have been attributable to other comorbidities.
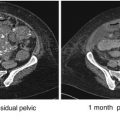
In the initial systematic study of metastatic low-grade serous carcinoma of the ovary, major features included relatively young age at diagnosis (median age = 43 years), prolonged overall survival (median OS = 82 months), and relative chemoresistance as reflected by the surrogate marker of persistent tumor at completion of primary treatment (48 % of patients) [89]. After adjusting for other variables, persistent disease after primary chemotherapy was associated with a shorter PFS time (HR = 2.64; P = 0.03). The theme of relative chemoresistance, thought to be related to the indolent nature of low-grade serous carcinoma, was subsequently also observed in reports of patients treated with neoadjuvant chemotherapy [90], patients with primary peritoneal low-grade serous carcinoma [92], and patients with recurrent disease [91]. Nevertheless, chemotherapy generally remains the standard therapy for women with stage II–IV low-grade serous carcinoma until such time that it is replaced by evidence-based alternative treatment. In addition, in the report of chemotherapy for recurrent low-grade serous carcinoma, 60 % of women had stable disease for a period of time. Whether the frequency of stable disease is more related to tumor biology or a therapeutic effect remains unresolved (Table 13.2).
Table 13.2
Molecular biomarkers and potential targets in low-grade serous carcinoma of the ovary or peritoneum
Gene or pathway | Frequency | Potential active agents | References |
|---|---|---|---|
KRAS | 20–40 % | MEKi | |
BRAF | 2–6 % | BRAFi | |
IGF-1R | Overexpressed compared to STLMP and HGSC | IGF-1Ri | [105] |
Angiogenesis (e.g., VEGF) | – | Antiangiogenesis agents (e.g., bevacizumab) | |
PI3K/AKT/mTOR | Rare | PI3Ki AKTi mTORi | [84] |
For some women, hormonal therapy may offer a greater benefit than chemotherapy with less associated toxicity [95]. In a report of 64 women with recurrent low-grade serous carcinoma who received 89 separate hormonal therapy regimens, 9 % of patient regimens resulted in an objective response, and 62 % of patient regimens resulted in stable disease [95]. In addition, ER/PR expression data were available in 50 patients in this study. Patients with ER+/PR- tumors had a shorter time to progression (HR = 1.8) than patients with ER+/PR+ tumors; however, this observation approached but did not reach statistical significance (p = 0.056). Thus, hormonal therapy remains a reasonable and potentially active treatment for women with metastatic low-grade serous carcinoma.
Given the realization that cytotoxic chemotherapy has limited activity in low-grade serous carcinoma, a search for more effective systemic therapies is warranted. As with most cancer types, investigators have principally focused on the study of targeted therapies over the past few years. Coupled with these efforts is the continued study of the molecular biology of low-grade serous carcinoma through additional basic science and translational research studies.
Targeted Therapeutics
Based on preclinical research findings, potential genes or pathways for targeting low-grade serous carcinoma include the MAPK pathway, IGF-1R, the angiogenesis pathway, and possibly the PI3K/AKT/mTOR pathway. The MAPK signaling pathway is one of the most activated and best characterized in cancer [109]. The MAPK cascade is triggered by the binding of a ligand that ultimately leads to phosphorylation of ERK [110, 111]. Thus, MEK is a good candidate for targeted therapy, and a number of MEKi have been developed in the past few years [112, 113]. Preclinical studies of ovarian cancer demonstrated significant growth inhibition in cell lines with KRAS or BRAF mutations compared with cell lines with wild-type cells [114, 115]. In view of the cumulative data indicating mutations within the MAPK pathway, as discussed above, exploration of MEKi in patients with low-grade serous carcinoma was a natural progression.
In a landmark GOG phase II trial (GOG 0239), Farley et al. demonstrated promising results with an MEKi, selumetinib [108]. Fifty-two women with recurrent low-grade serous carcinoma were enrolled in this trial and treated with the MEKi, selumetinib 50 mg twice daily. The overall response rate was 15 %, with one complete response and seven partial responses. Another 65 % of patients in the trial had stable disease. The median PFS was 11.0 months. The most common toxicities were gastrointestinal (13), dermatologic (nine), and metabolic (seven). Three patients experienced grade 4 toxicities—one each cardiac, pain, and pulmonary. Mutational analysis was conducted on formalin-fixed, paraffin-embedded tumor samples from 34 patients in this trial. The primary tumor accounted for 82 % of the cases. In these 34 cases, there were two (6 %) BRAF mutations and 14 (41 %) KRAS mutations. In this study, there was no correlation between mutations of BRAF or KRAS and objective response. Subsequently, the promising results of this trial in the context of the relatively low response rates of low-grade serous carcinoma to either chemotherapeutic or hormonal agents prompted further investigations.
Three ongoing phase II or III clinical trials have emerged from this experience. Each of these trials includes a different MEKi. The MILO trial (NCT01849874) is an open-label phase III protocol that randomizes patients with recurrent low-grade serous carcinoma to either chemotherapy (physician’s choice of pegylated liposomal doxorubicin, paclitaxel, or topotecan) or MEK162. A second trial (NCT01936363) has a randomized phase II design and includes the MEKi, pimasertib, with either placebo or SAR245409 (a PI3K/mTOR inhibitor). And GOG 0281 is a randomized phase II/III trial (NCT02101788) that has been activated through NRG Oncology. This trial includes a randomization between standard of care (physician’s choice of letrozole, tamoxifen, pegylated liposomal doxorubicin, weekly paclitaxel, or topotecan) and MEKi monotherapy, trametinib. This trial also includes a robust translational research component, with fresh and archival FFPE tissue for next-generation sequencing and proteomics as well as cell-free DNA and pharmacokinetic studies.
As noted above, the angiogenesis pathway may also be a target in patients with low-grade serous carcinoma. Bidus et al. reported three patients with apparent recurrent low-grade serous carcinomas (one with primary peritoneal low-grade serous carcinoma, one with ovarian low-grade serous carcinoma, and another with a mixed low-grade serous-endometrioid carcinoma) treated with bevacizumab [116]. All three patients experienced a sustained response—two partial responses and one complete response. Subsequently, Grisham et al. reported on 17 patients with low-grade serous carcinoma of the ovary or peritoneum who received bevacizumab [99]. Two patients were treated with single-agent bevacizumab and the others with a combination of bevacizumab and chemotherapy. Fifteen patients were evaluable for response, and six (40 %) had a partial response. An additional five (33.3 %) had stable disease lasting 3 months or longer.
To date, there have been no clinical trials exploring the role of IGF-1R targeted therapy in women with low-grade serous carcinoma. Likewise, although an agent targeting the PI3K/AKT/mTOR pathway in combination with an MEKi was administered to a proportion of women on one of the three trials above (NCT01936363), the results of this trial are pending, and no AKTi, PI3Ki, or mTORi monotherapy trials specifically for patients with low-grade serous carcinoma have been developed.
References
1.
Shih I-M, Kurman RJ. Ovarian tumorigenesis. Am J Pathol. 2010;164(5):1511–8. American Society for Investigative Pathology.
2.
Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28(4):496–504.PubMed
3.
Gershenson DM, Silva EG, Tortolero-Luna G, Levenback C, Morris M, Tornos C. Serous borderline tumors of the ovary with noninvasive peritoneal implants. Cancer. 1998;83(10):2157–63.PubMed
4.
Gershenson DM, Silva EG, Levy L, Burke TW, Wolf JK, Tornos C. Ovarian serous borderline tumors with invasive peritoneal implants. Cancer. 1998;82(6):1096–103.PubMed
5.
Crispens MA, Bodurka D, Deavers M, Lu K, Silva EG, Gershenson DM. Response and survival in patients with progressive or recurrent serous ovarian tumors of low malignant potential. Obstet Gynecol. 2002;99(1):3–10.PubMed
6.
Silva EG, Gershenson DM, Malpica A, Deavers M. The recurrence and the overall survival rates of ovarian serous borderline neoplasms with noninvasive implants is time dependent. Am J Surg Pathol. 2006;30(11):1367–71.PubMed
7.
Shvartsman HS, Sun CC, Bodurka DC, Mahajan V, Crispens M, Lu KH, et al. Comparison of the clinical behavior of newly diagnosed stages II-IV low-grade serous carcinoma of the ovary with that of serous ovarian tumors of low malignant potential that recur as low-grade serous carcinoma. Gynecol Oncol. 2007;105(3):625–9.PubMed
8.
Bonome T, Lee J-Y, Park D-C, Radonovich M, Pise-Masison C, Brady J, et al. Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res. 2005;65(22):10602–12. American Association for Cancer Research.PubMed
9.
Meinhold-Heerlein I, Bauerschlag D, Hilpert F, Dimitrov P, Sapinoso LM, Orlowska-Volk M, et al. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene. 2004;24(6):1053–65.
10.
Singer G, Kurman RJ, Chang H-W, Cho SKR, Shih I-M. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160(4):1223–8.PubMedPubMedCentral
11.
Singer G, Oldt R, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95(6):484–6.PubMed
12.
Singer G, Shih I-M, Truskinovsky A, Umudum H, Kurman RJ. Mutational analysis of K-ras segregates ovarian serous carcinomas into two types: invasive MPSC (low-grade tumor) and conventional serous carcinoma (high-grade tumor). Int J Gynecol Pathol. 2003;22(1):37–41.PubMed
13.
Taylor HC. Malignant and semimalignant tumors of the ovary. Surg Gynecol Obstet. 1929;48:206–30.
14.
Kottmeier HL, Kolstad P, mcGarrity KA. Annual report on results of treatment in gynecologic cancer, vol. 17. Statements of results obtained in 1969-1972, inclusive. FIGO. Stockholm: Editorial office, Radiumhemmet; 1973.
15.
Serov SF, Scully RE, Sobin LH. Histological typing of ovarian tumours, International histological classification of tumors, vol. 9. Geneva: World Health Organization; 1973.
16.
Skírnisdóttir I, Garmo H, Wilander E, Holmberg L. Borderline ovarian tumors in Sweden 1960-2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int J Cancer. 2008;123(8):1897–901. Wiley Subscription Services, Inc., A Wiley Company.PubMed
17.
Åkeson M, Zetterqvist BM, Dahllöf K, Jakobsen AM, Brännström M, Horvath G. Population-based cohort follow-up study of all patients operated for borderline ovarian tumor in western Sweden during an 11-year period. Int J Gynecol Cancer. 2008;18(3):453–9.PubMed
18.
duBois A, Ewald-Riegler N, de Gregorio N, Reuss A, Mahner S, Fotopoulou C, et al. Borderline tumours of the ovary: a cohort study of the Arbeitsgmeinschaft Gyna ̈kologische Onkologie (AGO) study group. Eur J Cancer. 2013;49(8):1905–14.
19.
Tropé CG, Kaern J, Davidson B. Borderline ovarian tumours. Best Pract Res Clin Obstet Gynaecol. 2012;26(3):325–36.PubMed
20.
Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. Risk factors for epithelial borderline ovarian tumors: results of a Swedish case–control study. Gynecol Oncol. 2001;83(3):575–85.PubMed
21.
Kolwijck E, Thomas CMG, Bulten J, Massuger LFAG. Preoperative CA-125 levels in 123 patients with borderline ovarian tumors. Int J Gynecol Cancer. 2009;19(8):1335–8.PubMed
22.
Seidman JD, Kurman RJ. Subclassification of serous borderline tumors of the ovary into benign and malignant types. A clinicopathologic study of 65 advanced stage cases. Am J Surg Pathol. 1996;20(11):1331–45.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree