| Infection | A pathologic process caused by invasion of normally sterile host tissue by pathogenic or potentially pathogenic microorganisms |
| Bacteremia | The presence of viable bacteria in the blood |
| SIRS | The systemic inflammatory response to a wide range of infectious and noninfectious conditions. Currently used criteria include two or more of the following: temperature >38°C or ≤36°C; heart rate >90 beats/min; respiratory rate >20 breaths/min, or PaCO2 ≤32 mm Hg; WBC >12 000 cells/mm3 or ≤4000 cells/mm3, or ≥10% immature (band) forms |
| Sepsis | The clinical syndrome defined by the presence of both infection and a systemic inflammatory response |
| Severe sepsis | Sepsis complicated by organ dysfunction, hypotension, or signs of hypoperfusion (e.g., lactic acidosis, renal failure, altered mental status, and acute respiratory failure) |
| Septic shock | Sepsis accompanied by acute circulatory failure, characterized by persistent arterial hypotension that, despite adequate fluid resuscitation, requires pressor therapy |
| MODS | Multiple organ dysfunction syndrome; the presence of altered organ function in an acutely ill patient such that homeostasis cannot be maintained without intervention; primary multiple organ dysfunction syndrome is the direct result of a well-defined insult in which organ dysfunction occurs early and can be directly attributable to the insult itself; secondary multiple organ dysfunction syndrome develops as a consequence of a host response and is identified in the context of SIRS |
Abbreviations: SIRS = systemic inflammatory response syndrome; MODS = multiple organ dysfunction syndrome; WBC = white blood cells.
| Infection (documented or suspected) and some of the following parameters must be present: | |
|---|---|
| General parameters | (1) Fever (>38°C), (2) hypothermia (core temperature <36°C), (3) heart rate>90 beats/min or > than 2 SDs above the normal value, (4) tachypnea, (5) altered mental status, (6) significant edema or positive fluid balance (>20 mL/kg over 24 hours), (7) hyperglycemia (plasma glucose>140 mg/dL or 7.7 mmol/L) in the absence of diabetes |
| Inflammatory parameters | (1) Leukocytosis (white blood cell count >12 000/μL), (2) leukopenia (white blood cell count <4000/μL), (3) normal white blood cell count with >10% immature forms, (4) plasma C-reactive protein (CRP) > than 2 SDs above the normal limit, (5) plasma procalcitonin > 2 SDs above the normal limit |
| Hemodynamic parameter | (1) Arterial hypotension (SBP <90 mm Hg, MAP <70 mm Hg, or an SBP decrease of >40 mm Hg in adults or >2 SDs below normal for age |
| Organ dysfunction parameters | (1) Arterial hypoxemia (PaO2/FiO2 <300), (2) acute oliguria (urine output <0.5 mL/kg/h for at least 2 hours despite adequate fluid resuscitation), (3) creatinine increase >0.5 mg/dL or 44.2 μmol/L, (4) coagulation abnormalities (INR >1.5 or aPTT >60 s), (5) ileus (absent bowel sounds), (6) thrombocytopenia (platelet count <100 000/μL), (7) hyperbilirubinemia (plasma total bilirubin >4 mg/dL or 70 μmol/L) |
| Tissue perfusion parameters | (1) Hyperlactatemia (>1 mmol/L), (2) decreased capillary refill or mottling |
Abbreviations: SBP = systolic blood pressure; MAP = mean arterial pressure; SD = standard deviation; INR = international normalized ratio; aPTT = activated partial thromboplastin time.
In the pediatric population, the diagnostic criteria for sepsis are: (1) signs and symptoms of inflammation plus (2) infection with (3) hyper- or hypothermia (rectal temperature >38.5°C or <35°C), (4) tachycardia (may be absent in hypothermic patients), and at least one of the following indications of altered organ dysfunction – (a) altered mental status, (b) hypoxemia, (c) increased serum lactate level, or (d) bounding pulses.
Epidemiology
The incidence of sepsis, severe sepsis, and septic shock are probably underestimated since most estimates are based on hospital databases that rely on the International Classification of Diseases, and so are biased toward a more severely ill population. The global incidence of sepsis is reported as from 22 to 240 cases/100 000 persons; severe sepsis from 13 to 300 cases/100 000 persons; and for septic shock, 11 cases/100 000 persons (based on a 2012 study). Case-fatality rates are as high as 30% for sepsis, 50% for severe sepsis, and 80% for septic shock. In the United States, the incidence of severe sepsis had been rising but in-hospital mortality was decreasing and not significantly different from Europe.
The elderly, neutropenic patients, and infants have higher attack and mortality rates. The incidence appears to be higher in nonwhites and men for unknown reasons. However, women admitted to ICUs for severe sepsis had higher risk of death, possibly due to gender-associated bias for men as to the level of care given. Age-adjusted case-fatality rates are similar for hospitalized septic whites and blacks, but blacks have higher rates of hospitalization and population-based mortality for sepsis. No differences in quality of care between groups exist, but there may be disparities in preventive medicine and care of pre-existing conditions in blacks.
Pathogenesis
The clinical manifestations of the sepsis syndrome are caused by the body’s immune, inflammatory, and coagulation responses to toxins and other components of microorganisms. Endotoxin, the lipoidal acylated glucosamine disaccharide core of the cell wall of many aerobic gram-negative bacteria, starts the cascade of inflammatory mediators. Known as lipid A, it is highly conserved in Enterobacteriaceae and in Pseudomonaceae. Anaerobic gram-negative bacteria, such as Bacteroides fragilis, lack lipid A, perhaps explaining why sepsis is not as common when infection is caused by anaerobes.
Once a pathogenic organism invades a host barrier, its cell wall molecules (lipid A in gram-negative bacteria; and peptidoglycan, teichoic acid, or toxic shock toxin-1 [TSST-1] in gram positives) are sensed by local defense cells expressing specific host proteins on their surface, such as CD14 and toll-like receptors (TLRs). The peptidoglycan of gram-positive bacteria is recognized by TLR2, and the lipopolysaccharide (LPS) of gram negatives by TLR4. Activation of TLRs initiates intracellular signaling pathways. Subsequently, macrophages are activated, leading to the rapid production (minutes to hours) of cytokines and immunomodulatory molecules that have potent biologic effects and mediate an inflammatory response.
Tumor necrosis factor-α (TNF-α) is the most important cytokine in sepsis. Interleukin (IL)-1β has similar effects. The two stimulate the release of stress hormones, other cytokines (e.g., IL-2, IL-6, IL-8, IL-10), and other inflammatory mediators of sepsis (e.g., nitric oxide, lipoxygenase and cyclooxygenase metabolites, platelet activation factor, interferon-γ, and adhesion molecules). All of these interact in a complex fashion to effect the various changes to multiple organ systems.
The resulting sepsis, multiorgan system failure, and death were previously believed to be solely from an exaggerated, uncontrolled inflammatory response, known as “cytokine storm.” But it is now thought that they also arise from subsequent immunosuppression – the result of an “injured” adaptive immune response. This state includes the alteration of neutrophil migration at multiple stages, as these cells become more rigid and sequestered in organs (limiting blood flow, causing tissue ischemia and multiorgan failure), as well as suppressed because of reduced TLR expression and signaling. Nitric oxide blocks neutrophil migration and the interaction between leukocytes and endothelial cells. Likewise, peroxisome proliferator-activated receptor (PPAR)-γ contributes to neutrophil chemotaxis suppression. There is also evidence that gene expression of TNF-α and interferon-β stops with continued insult, necrosis, and infection. The initial release of, and exposure to, high levels of chemoattractants “desensitizes” G-protein-coupled receptor (GPCR) responsiveness, resulting in downregulation of GPCR cell surface expression. All of these effects show that the innate immune system seems unable to respond to continuous inflammatory stimuli, progressing to a dysfunctional stage and ending in an irreversible phase of sepsis and end-organ injury (Figure 2.1).
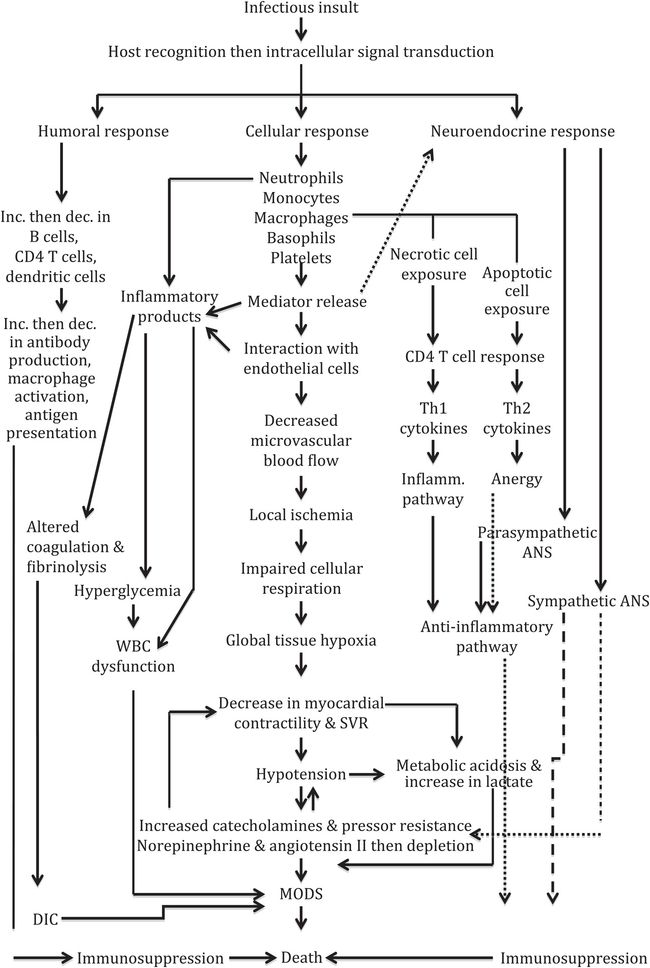
Figure 2.1 Comprehensive flow chart on the pathophysiology of severe sepsis/septic shock. Abbreviations: ANS = autonomic nervous system; dec. = decrease; DIC = disseminated intravascular coagulation; inc. = increase; inflamm. = inflammatory; MODS = multiorgan dysfunction syndrome; SVR = systemic vascular resistance; Th = T helper cell.
Etiology
Historically, antibiotic recommendations for therapy of sepsis and septic shock were based on coverage of gram-negative organisms. However, sepsis caused by gram-positive organisms is clinically identical to sepsis caused by gram negatives. After 1987, gram-positive bacteria became the predominant pathogens in most areas. In recent studies, 47% to 55% of sepsis cases were due to gram positives (e.g., Staphylococcus aureus, coagulase-negative staphylococci, Streptococcus pneumoniae, and enterococci), while gram negatives made up 38–51%. Escherichia coli has remained the most common gram-negative pathogen in community and nosocomial infections. Staphylococcus epidermidis has become the most common cause of nosocomial bacteremias, followed by S. aureus, enterococci, and Candida species. Infections caused by vancomycin-resistant enterococci (VRE), particularly Enterococcus faecium (resistant to ampicillin and aminoglycosides) and non-albicans Candida species have become more common. Gram-negative bacteria, including multidrug-resistant (MDR) Pseudomonas aeruginosa, extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, Enterobacter species and other plasmid-mediated AmpC β-lactamase producing bacteria, and Acinetobacter species, are increasing and becoming resistant to multiple antibiotics.
Diagnosis
Sepsis should be considered when a patient displays symptoms and signs of systemic inflammation in response to infection. The diagnostic criteria are in Table 2.2. Prompt administration of empiric antibiotics is appropriate, but every attempt should be made to determine the source, microbiology, and pathophysiology of the infection so as to guide optimal management. Often, various underlying risk factors predispose individuals to infection with specific organisms. Some of these conditions and associated pathogens are in Table 2.3.
| Circumstance | Possible pathogens |
|---|---|
| Splenectomy (traumatic or functional) | Encapsulated organisms: Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis |
| Neutropenia (≤500 neutrophils/μL) | Gram-negatives, including Pseudomonas aeruginosa; gram-positives, including Staphylococcus aureus; fungi, especially Candida species |
| Hypogammaglobulinemia (e.g., CLL) | Streptococcus pneumoniae, Escherichia coli |
| Burns | MRSA, Pseudomonas aeruginosa, resistant gram-negatives, Candida species |
| AIDS | Pseudomonas aeruginosa (if neutropenic), Salmonella species, Staphylococcus aureus, Pneumocystis jirovecii (pneumonia) |
| Intravascular devices | Staphylococcus aureus, Staphylococcus epidermidis |
| Nosocomial infections | MRSA, Staphylococcus epidermidis, Enterococcus species, resistant gram-negatives, Candida species |
Abbreviations: CLL = chronic lymphocytic leukemia; AIDS = acquired immunodeficiency syndrome; MRSA = methicillin-resistant Staphylococcus aureus.
A thorough history and physical examination are crucial. Multiple cultures from suspected infected sites need to be obtained. All culture specimens should be delivered promptly to the microbiology laboratory. Gram staining should be done and read as soon as possible on specimens submitted for culture. Ideally, cultures should be obtained before starting antibiotics. Any significant delay (>45 minutes) in antimicrobial administration should be avoided. For clinically or hemodynamically unstable patients, antibiotics should be given immediately.
At least two sets of blood cultures from different sites should be obtained from all sepsis suspects. Each blood culture set consists of one aerobic and one anaerobic bottle. Typically, at least 10 mL of blood needs to be injected into each bottle to increase the likelihood of culturing pathogens. If an indwelling venous or arterial catheter is present, it is important to obtain additional cultures through each port, unless the device was just recently placed (<48 hours). If the culture set taken from the line becomes positive much earlier (>2 hours faster) than the one from the peripheral vein, then the vascular access site is more likely to be the source of infection. Quantitative cultures of the catheter and the peripheral blood may be used when seeking more evidence for or against catheter-associated infection. The catheter is the likely source if it has significantly more bacterial colonies versus the peripheral blood.
Sputum for culture can be spontaneously expectorated, induced with 3% saline, or obtained by nasotracheal, endotracheal, or transtracheal techniques. Specimens should have ≥25 polymorphonuclear cells and ≤10 squamous epithelial cells per low-power microscopic field to decrease the chance that the specimen is contaminated with upper airway flora. Semi-quantitative or quantitative cultures may be used for ventilator-associated pneumonia (VAP) diagnosis.
Urine should be obtained for culture when the suspected source of infection is the urinary tract. Clean-catch or straight-catheterization specimens are preferred. Urine that has been present in a closed collection system for more than 1 hour should not be sent for culture. If necessary, urine can be obtained directly from the catheter tubing or bladder (suprapubic aspiration) using a syringe and a small-gauge needle. Remember that many bacteriuric patients, especially those with indwelling urinary catheters, may be septic from another source. The presence of >100 000 bacteria/mL in urine culture suggests infection; however, this criterion has been validated only for ambulatory young women with gram-negative bacteria. Cultures from other sites should be obtained if clinically indicated.
Order an initial lactate at the Emergency Department (ED) and repeat it in 6 hours to compute the 6-hour lactate clearance (shown to be a predictor of mortality), since 36% clearance may be an appropriate resuscitation end point.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree