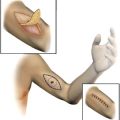
The technique of lymphatic mapping and sentinel lymph node (SLN) biopsy was introduced 20 years ago as an important advance in the management of patients with stage I and II melanoma. After 2 decades of experience, SLN biopsy and the practice of selective lymphadenectomy represents a minimally invasive standard of care that facilitates the accurate staging of the clinically negative regional lymph node basin, provides durable regional disease control, and improves survival in node-positive patients.
The increasing incidence of cutaneous melanoma over the past few decades has created the need as well as the opportunity to establish standards of surgical care designed to achieve the following goals: (1) accurate staging and prognosis, (2) long-term locoregional disease control, and (3) optimizing the chance for cure. Establishing how best to manage the clinically negative regional lymph node basin(s) in newly diagnosed American Joint Committee on Cancer (AJCC) stage I and II patients has been identified as one of the strategies to fulfill these goals and has been the focus of intense global clinical research efforts. Significant emphasis has also been placed on accomplishing these goals while minimizing treatment-related morbidities. The technique of lymphatic mapping and sentinel lymph node (SLN) biopsy, first presented in 1990 and published in 1992 by Morton and colleagues, was introduced as a minimally invasive method to accurately stage the clinically negative regional nodal basins in patients with stage I and II melanoma, and fostered adoption of a selective approach to formal lymphadenectomy in the treatment of microscopic lymph node metastases. Now recognized as a paradigm-changing landmark approach, global interest developed quickly for this rational management strategy. Several confirmatory studies, including completion of prospective randomized trials, have together generated a wealth of information regarding many issues related to the use of SLN biopsy, including accuracy, prognostic value, selection of appropriate candidates, use of enhanced histologic techniques, novel lymphatic drainage imaging studies, impact on regional disease control, and impact on morbidity and survival. While this technique has been widely regarded as one of the most important advances ever made in melanoma treatment, several controversies have nonetheless emerged.
On the eve of the twentieth anniversary since the introduction of SLN biopsy for patients with early-stage melanoma, it is appropriate and relevant to review the lessons learned and to critically evaluate the published SLN biopsy experience to assess its current role in the management of patients with stage I and II melanoma, to discuss some of the major controversies, and to outline likely future directions.
The evolution of SLN biopsy as a rational management strategy in patients with AJCC stage I and II melanoma
The surgical approach in patients with stage I and II melanoma is often considered thematically by addressing 2 principles: (1) wide excision of the primary melanoma and (2) approach to regional lymph node evaluation. Whereas recommendations for the extent of excision margins are largely based on clinical trials and are therefore well established and widely accepted, the approach to patients with clinically uninvolved regional lymph nodes has been the subject of extensive debate and ongoing controversy. The following clinical vignette further illustrates this clinical debate. An otherwise healthy 36-year old patient noted a changing pigmented lesion over the left scapula, and following narrow excisional biopsy of this site, was diagnosed with a 1.8-mm nonulcerated melanoma. On presentation for further evaluation and treatment planning, physical examination revealed no other suspicious cutaneous lesions and no palpable regional lymph nodes in any potential regional lymph node group. A chest radiograph was normal.
Historically, in addition to a recommendation for wide excision of the primary tumor, this patient would have likely been offered one of two approaches to the regional nodal basin: (1) nodal observation followed by formal lymph node dissection only if clinically evident (ie, palpable) nodal disease subsequently developed, a procedure termed therapeutic lymph node dissection (TLND), or (2) formal lymph node dissection as a component of the initial surgical treatment, commonly referred to as elective lymph node dissection (ELND). Although a detailed discussion is beyond the scope of this article, both approaches have theoretical and real disadvantages.
A significant fraction of patients with melanoma, predicted by primary tumor and other factors, including increasing primary tumor thickness, ulceration, high mitotic rate, or other unfavorable histologic features of the primary tumor, harbor clinically undetectable regional lymph node metastases at the time of presentation. Evolving data indicate that such microscopic regional disease will ultimately lead to palpable (ie, macroscopic) nodal disease. Once clinical nodal involvement develops, however, the ability to achieve long-term survival and durable regional disease control with a TLND may be compromised as compared with surgical approaches targeted at treating microscopic nodal burden. The clinical challenge is that following TLND, the rates of distant metastatic disease and relapse in the treated nodal basin are at least 50% and 15% to 50%, respectively.
Of importance, the practice of ELND was popularized with the intent of reducing these high rates of disease recurrence. Proponents of ELND suggested that removal of microscopically involved lymph nodes would prevent the development of clinically apparent lymph node metastasis and, at least in theory, eliminate a potential source of distant failure. Furthermore, regional lymph node surgery performed when the lymph node disease burden is microscopic and when few nodes are involved would minimize the risk of clinical recurrence in the affected basin, and potentially spare the patient from tumor burden–related sequelae including pain, skin ulceration, blood vessel and nerve involvement, and advanced lymphedema that can be associated with this pattern of recurrence.
In the majority of patients with clinically node-negative primary cutaneous melanoma, microscopic nodal disease is fortunately absent at diagnosis. It follows that such patients cannot benefit from an ELND; however, if ELND is performed, patients would be subjected to the cost and morbidity of an unnecessary operation. Because only approximately 20% of newly diagnosed stage I and II melanoma patients are considered to have an intermediate or high risk of harboring occult regional nodal disease, it is not surprising that overall survival advantages were not observed in prospective randomized trials comparing ELND to nodal observation in stage I and II patients predicted to have an intermediate or high risk for harboring microscopic regional nodal disease. As a result, the routine practice of ELND was appropriately challenged.
Stemming in part from this controversy, and formally introduced and detailed in the next section, a rational strategy emerged when the technique of lymphatic mapping and SLN biopsy was introduced as a minimally invasive method for identifying patients who harbor occult nodal microscopic metastases. Patients with proven occult regional nodal disease in the SLN could undergo an “early” TLND, and the majority of patients without nodal disease could be safely observed. This approach, termed selective lymphadenectomy, has been extensively studied worldwide and has gained widespread global use.
Scientific support for the sentinel node concept
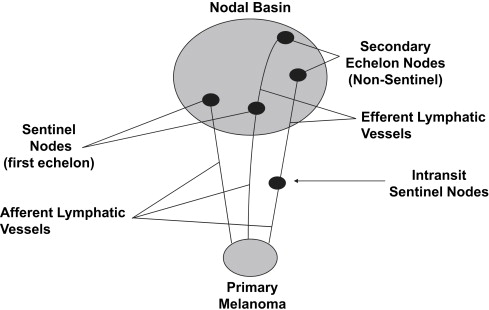
Lymphatic mapping relies on the hypothesis that the dermal lymphatic drainage from cutaneous sites to the regional lymph node basin(s) occurs as an orderly and definable process, and that these lymphatic drainage patterns should mimic or predict the potential routes of metastatic spread of melanoma cells via the lymphatics to regional lymph nodes ( Figs. 1 , 2 A, and 3 ). Accordingly, the first lymph node(s) receiving direct afferent lymphatic drainage (ie, the SLNs) are the most likely to contain metastatic disease if any regional lymph nodes are involved. It then follows that successful identification, surgical removal, and careful histologic examination of all SLNs in a patient should provide accurate regional nodal staging.
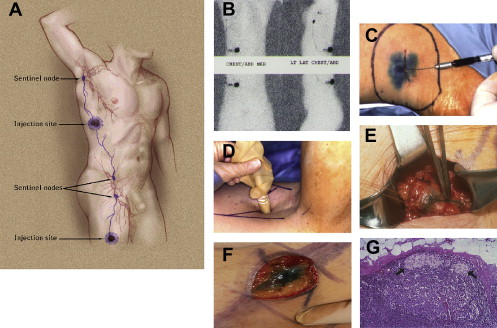
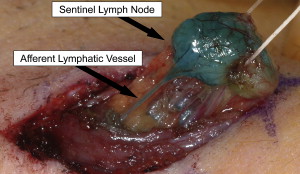
To test this hypothesis, clinical studies were performed using intradermal injections of blue dyes (isosulfan blue or patent blue V) around the primary tumor site that are taken up and transported via the lymphatics (essentially “staining” the lymphatic fluid blue), followed by the visual identification and removal of SLNs in the regional nodal basin(s). These studies established the following: (1) SLN identification rates and (2) the accuracy of the SLN in determining the presence or absence of regional nodal metastases.
The landmark study that formally introduced this concept to the melanoma community was published by Donald Morton’s group in 1992; among 237 evaluated patients, the SLN identification rate was 82%. Subsequent studies from the University of Texas MD Anderson Cancer Center, the Sydney Melanoma Unit, and Moffitt Cancer Center reported similar findings. In the early 1990s, accuracy was assessed through the use of synchronous ELND performed at the time of the SLN biopsy procedure. A false-negative event was defined as the detection of microscopic disease in non-SLNs when the SLN(s) from the same basin was histologically negative. Accordingly, the false-negative rate was then calculated as the number of false-negative events divided by the total number of patients with microscopic nodal disease. These initial studies collectively evaluated 402 patients with successful SLN localization, 86 of whom were found to have regional node metastases (81 patients with a positive SLN and 5 additional patients with disease only in a non-SLN). This low false-negative rate of approximately 5% supported the SLN concept.
Additional evidence that regional node metastasis constitutes an orderly and nonrandom event was provided from an MD Anderson study that examined 105 lymphadenectomy specimens in patients with at least one positive SLN. Investigators found that the SLN was the only lymph node involved in 83 (79%) of the basins, with microscopic nodal metastasis identified in an additional 21% of the lymphadenectomy specimens. Overall, among these patients with at least one positive SLN, 68% of all the SLNs harvested and only 1.8% of all non-SLNs removed contained metastatic disease.
Tremendous interest was generated by these initial studies, and many centers subsequently adopted the selective lymphadenectomy approach for newly diagnosed intermediate- and high- risk stage I and II melanoma patients. Improvements in SLN localization techniques, insights into the biologic relevance of the SLN (see later discussion), and additional findings supporting the SLN concept emerged. In a report of nearly 250 SLN-negative patients followed for over 3 years, only 10 patients (4%) developed subsequent nodal failure within the previously mapped regional lymph node basins. Such failures represent a false-negative rate similar to the 5% determined by concomitant ELND. Of note, this study represented patients who had SLN biopsy early in the global experience, when individual SLNs were subjected only to “routine” histologic analysis. More intense histologic scrutiny of the “negative” SLNs from these same 10 patients as part of this study revealed the presence of disease in 8 patients (8/10). These data not only further supported the validity of the SLN concept but also suggested that routine histologic examinations of SLNs may occasionally fail to detect clinically relevant disease.
Scientific support for the sentinel node concept
Lymphatic mapping relies on the hypothesis that the dermal lymphatic drainage from cutaneous sites to the regional lymph node basin(s) occurs as an orderly and definable process, and that these lymphatic drainage patterns should mimic or predict the potential routes of metastatic spread of melanoma cells via the lymphatics to regional lymph nodes ( Figs. 1 , 2 A, and 3 ). Accordingly, the first lymph node(s) receiving direct afferent lymphatic drainage (ie, the SLNs) are the most likely to contain metastatic disease if any regional lymph nodes are involved. It then follows that successful identification, surgical removal, and careful histologic examination of all SLNs in a patient should provide accurate regional nodal staging.
To test this hypothesis, clinical studies were performed using intradermal injections of blue dyes (isosulfan blue or patent blue V) around the primary tumor site that are taken up and transported via the lymphatics (essentially “staining” the lymphatic fluid blue), followed by the visual identification and removal of SLNs in the regional nodal basin(s). These studies established the following: (1) SLN identification rates and (2) the accuracy of the SLN in determining the presence or absence of regional nodal metastases.
The landmark study that formally introduced this concept to the melanoma community was published by Donald Morton’s group in 1992; among 237 evaluated patients, the SLN identification rate was 82%. Subsequent studies from the University of Texas MD Anderson Cancer Center, the Sydney Melanoma Unit, and Moffitt Cancer Center reported similar findings. In the early 1990s, accuracy was assessed through the use of synchronous ELND performed at the time of the SLN biopsy procedure. A false-negative event was defined as the detection of microscopic disease in non-SLNs when the SLN(s) from the same basin was histologically negative. Accordingly, the false-negative rate was then calculated as the number of false-negative events divided by the total number of patients with microscopic nodal disease. These initial studies collectively evaluated 402 patients with successful SLN localization, 86 of whom were found to have regional node metastases (81 patients with a positive SLN and 5 additional patients with disease only in a non-SLN). This low false-negative rate of approximately 5% supported the SLN concept.
Additional evidence that regional node metastasis constitutes an orderly and nonrandom event was provided from an MD Anderson study that examined 105 lymphadenectomy specimens in patients with at least one positive SLN. Investigators found that the SLN was the only lymph node involved in 83 (79%) of the basins, with microscopic nodal metastasis identified in an additional 21% of the lymphadenectomy specimens. Overall, among these patients with at least one positive SLN, 68% of all the SLNs harvested and only 1.8% of all non-SLNs removed contained metastatic disease.
Tremendous interest was generated by these initial studies, and many centers subsequently adopted the selective lymphadenectomy approach for newly diagnosed intermediate- and high- risk stage I and II melanoma patients. Improvements in SLN localization techniques, insights into the biologic relevance of the SLN (see later discussion), and additional findings supporting the SLN concept emerged. In a report of nearly 250 SLN-negative patients followed for over 3 years, only 10 patients (4%) developed subsequent nodal failure within the previously mapped regional lymph node basins. Such failures represent a false-negative rate similar to the 5% determined by concomitant ELND. Of note, this study represented patients who had SLN biopsy early in the global experience, when individual SLNs were subjected only to “routine” histologic analysis. More intense histologic scrutiny of the “negative” SLNs from these same 10 patients as part of this study revealed the presence of disease in 8 patients (8/10). These data not only further supported the validity of the SLN concept but also suggested that routine histologic examinations of SLNs may occasionally fail to detect clinically relevant disease.
Histologic examination of SLNs
The fundamental goal of SLN biopsy is to accurately stage the regional lymph node basin, and is accomplished by the accurate identification and complete surgical removal of all the SLNs from the appropriate nodal basins at risk, followed by careful histologic examination of these SLNs. Although the definition of careful histologic examination continues to evolve, it is clear that it is logistically more feasible to apply sensitive techniques such as multiple sections or immunohistochemical analysis to a few lymph nodes (ie, the SLNs), rather than to the 20 to 30 lymph nodes submitted following a formal ELND. By carefully evaluating the nodes most likely to contain metastatic disease—the SLNs—more accurate nodal staging is possible, and with little morbidity to the patient.
Historically, the standard approach for evaluating lymph nodes, and therefore initially applied to the evaluation of SLNs as well, was to bivalve each clinically negative node and evaluate a section from each half using hematoxylin and eosin (H&E) staining. As a result, only a very small percentage of each lymph node(s) was actually sampled, and this likely explains why conventional histologic techniques underestimated the incidence of regional nodal disease in stage I and II melanoma patients. For example, the incidence of nodal failure following wide excision alone (ie, nodal observation) for primary melanomas 2 to 4 mm in thickness is approximately 35% to 50%, whereas the incidence of microscopic nodal disease as determined by ELND or SLN biopsy specimens, as determined by routine pathologic technique (ie, bivalving the nodes), is approximately 25% to 40%. While subsequent nodal failure may in part result from clinically occult in-transit disease, several lines of evidence support the concept that nodal disease is more often present at diagnosis than is demonstrated by routine conventional histology: (1) step-sectioning of the SLN (ie, better sampling) increases detection of microscopic disease, (2) up to 80% of patients who recur in the mapped nodal basin after a negative SLN biopsy initially assessed by routine pathology are determined to be node-positive following more careful analysis of the SLN paraffin blocks, and (3) evaluation of SLNs using reverse transcriptase–polymerase chain reaction (RT-PCR) to detect the presence of messenger RNA encoding melanoma-related proteins (eg, tyrosinase) as potential surrogate markers for the presence of melanoma cells results in higher SLN-positive rates. Studies demonstrate that essentially all H&E-positive SLNs and approximately 25% to 50% of H&E-negative SLNs are PCR-positive. It is intriguing that although preliminary clinical correlation studies demonstrated that recurrence rates for the PCR-positive/H&E-negative group were intermediate between the PCR-negative and H&E-positive patients, longer-term follow-up in prospective studies from the Sunbelt Melanoma Trial and Memorial Sloan-Kettering Cancer Center each failed to demonstrate an overall decreased survival in the PCR-positive/H&E-negative patients compared with the PCR-negative/H&E-negative patients. As histologic techniques become more sensitive, specificity may be compromised, but the more careful and complete the evaluation of SLNs, the more likely that a truly homogeneous SLN-negative subset can be defined.
Current recommendations include multiple H&E sections (ie, step-sectioning) and “routine” use of immunohistochemistry using HMB-45 and MART-1 antibodies; established standards continue to evolve and are detailed elsewhere in this issue (see article on melanoma pathology by Scolyer and Prieto). Frozen section analysis at the time of SLN biopsy probably reduces the sensitivity and is therefore not recommended, but imprint touch cytology performed on multiple sections of the SLN at the time of the SLN procedure can accurately detect microscopic disease in a significant fraction of patients with occult metastases, and may facilitate same-day completion lymphadenectomy without compromising the formal permanent histologic examination in patients who are SLN-positive and for whom concomitant completion lymphadenectomy was discussed preoperatively. RT-PCR evaluation or other molecular-based studies is currently appropriate only in the context of a clinical trial.
Technical advances
Initial SLN identification rates of up to 85% using blue dye injections alone provided early promise for this technique. The use of high-resolution cutaneous lymphoscintigraphy and an intraoperative hand-held gamma detection device to locate focal accumulation of radiotracer in SLNs following intradermal injection around the primary site have together dramatically improved SLN identification rates. The use of an intraoperative gamma probe was first described by Krag and colleagues, who reported a 95% SLN identification rate using this technique. Studies comparing combined modality techniques (ie, radiocolloid plus blue dye) to blue dye alone demonstrated a significant increase in SLN identification (to 99%) with the combined approach. Furthermore, Gershenwald and colleagues from MD Anderson observed that the number of SLNs identified was greater when both modalities were employed as compared with blue dye alone (1.74 vs 1.31, respectively). Overall, intraoperative use of the gamma probe provides an independent and complementary method of detection that is more sensitive for SLN identification than visualization of blue-stained nodes alone, and facilitates localization of SLNs that may otherwise go undetected during the SLN biopsy procedure. It follows, therefore, that more complete identification and removal of SLNs using the combined modality approach may further reduce an already low false-negative rate (see Fig. 2 ).
These techniques also facilitate localization of SLNs that may exist outside of the formal named nodal basins; such SLNs are commonly referred to as unusual, interval, in-transit, or ectopic SLNs ( Fig. 4 ). The incidence of SLNs in such locations is approximately 5% to 10%; interestingly, the frequency of microscopic involvement in such SLNs is similar to that observed in SLNs harvested from formal named major basins. The failure to identify these SLNs risks understaging some patients and leaving behind potential sources of clinical recurrences. More recently, the use of SPECT/CT (a fusion imaging technique of nuclear [single-photon emission] and computed tomography images) provides enhanced and 3-dimensional spatial resolution of areas of increased focal radiotracer uptake activity that correspond to SLNs, and is particularly helpful in identifying the anatomic location of SLNs in the head and neck region.
Biologic and prognostic significance of the sentinel node
Studies have demonstrated that the incidence of SLN metastases correlates directly with increasing tumor thickness. SLN involvement is also associated with a variety of other known primary tumor factors predictive of survival, including ulceration, lymphatic invasion, mitotic rate, Clark level, anatomic site, and host factors such as age. In a multivariate analysis by Rousseau and colleagues of 1375 patients with primary melanoma who underwent SLN biopsy, tumor thickness and the presence of ulceration both independently predicted SLN involvement ( Table 1 ). Of note, the 2002 AJCC melanoma staging committee database analysis demonstrated that the same 2 factors were also the strongest predictors of survival in stage I and II patients. This analysis uncovered a unique interaction between tumor thickness and ulceration in that the presence of ulceration within a specific tumor thickness stage worsened the prognosis of patients equivalent to those in the next higher thickness group without ulceration. A similar relationship was also observed between thickness and ulceration in terms of predicting the incidence of SLN metastases. These observations support the hypothesis that the prognostic value of tumor thickness and ulceration is largely dependent on the fact that these 2 same factors predict SLN metastases, and in this way provide convincing evidence that SLN involvement is a biologically important event. As noted in the article elsewhere in this issue on staging and prognosis by Dickson and Gershenwald, mitotic activity has been shown to be an important independent predictor of survival, and at a cut point of 1/mm 2 has recently been incorporated into the new AJCC seventh edition definition of T1b primary melanoma. It is interesting that preliminary data also indicate that SLN involvement correlates with mitotic activity for the subset of patients with a primary melanoma less than 1 mm in thickness.
| Tumor Thickness (mm) | Total Patients (%) | Positive SLN | |||||
|---|---|---|---|---|---|---|---|
| All (%) | Not Ulcerated | Ulcerated | P Value a Ulcerated vs Not Ulcerated | ||||
| (%) | AJCC Stage b | (%) | AJCC Stage b | ||||
| ≤1.00 | 28 | 4 | 3 | IA | 16 | IB | .026 |
| 1.01–2.00 | 38 | 12 | 11 | IB | 22 | IIA | .007 |
| 2.01–4.00 | 23 | 28 | 25 | IIA | 34 | IIB | .115 |
| >4.00 | 11 | 44 | 33 | IIB | 53 | IIC | .021 |
| All patients | 100 | 17 | 12 | 35 | <.0001 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree